Figure 4.
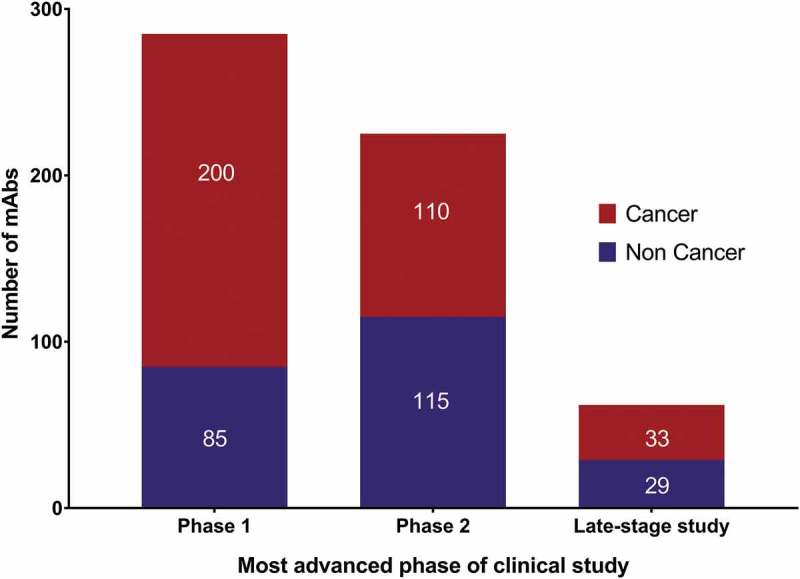
Clinical phases for antibody therapeutics in development.
Data as of November 2018. Totals include only antibody therapeutics sponsored by commercial firms; those sponsored solely by government, academic or non-profit organizations were excluded; biosimilars and Fc fusion proteins were excluded. Phase 1/2 included with Phase 2; late-stage studies include pivotal Phase 2, Phase 2/3 and Phase 3. Tables of mAbs in late-stage studies are available at www.antibodysociety.org.
