Abstract
Apatinib (Jiangsu HengRui Medicine Co. Ltd), a vascular endothelial growth factor receptor 2 (VEGFR-2) tyrosine kinase inhibitor, has been proven to be safe and to significantly prolong survival in advanced chemotherapy-refractory gastric cancer. This study aimed to assess and compare the efficacy and safety of apatinib combined with chemotherapy with that of chemotherapy alone as second- or higher-line treatment in patients with advanced and metastatic gastric or those with metastatic gastroesophageal junction adenocarcinoma (mGC).
Patients with chemotherapy-refractory mGC at Jiangsu Cancer Hospital & Research Institute were prospectively enrolled and assigned into 2 groups at a 2:1 ratio. The first group (combination group) comprised patients with combination treatment (apatinib + chemotherapy), while the second group comprised patients treated with chemotherapy alone (chemotherapy group). The dose of apatinib was 500 mg/d, and the chemotherapy regimens were based on fluoropyrimidine, platinum, and paclitaxel or irinotecan. The primary end points were progression-free survival (PFS).
Between November 2014 and December 2016, 175 patients were enrolled. PFS was significantly improved in the combination group compared with that in the chemotherapy group (8.5 months [95% confidence interval [CI], 6.45–10.54] vs 7.0 months [95% CI, 5.12–8.88] P = .021; hazard ratio (HR): 0.645 [95% CI: 0.429–0.969] P = .035). The disease control rate (DCR) was also higher in the combination group than that in the chemotherapy group (58.4% vs 41.9%, P = .041). Moreover, the incidence of Grade 3 to 4 hand-foot syndrome, proteinuria, and hypertension was significantly different between the 2 groups. Combined therapy (P = .040) and metastatic sites <2 (P = .008) were the independent prognostic factors for disease progression.
Compared with chemotherapy alone, the addition of apatinib to chemotherapy could better improve PFS and DCR with an acceptable safety profile for mGC refractory to 1 or more line of prior chemotherapy.
Keywords: apatinib, chemotherapy, efficacy, gastroesophageal junction adenocarcinoma, progression-free survival, toxicity, vascular endothelial growth factor
1. Introduction
Gastric cancer is the third leading cause of cancer-related death in Asian patients[1] because it is usually diagnosed at an advanced and metastatic stage. First- and second-line chemotherapy provide survival advantage compared with best supportive care for metastatic gastroesophageal junction adenocarcinoma (mGC).[2,3] Third-line chemotherapy with irinotecan or taxol have also been to be effective for gastric cancer.[22] However, salvage chemotherapy after failure of first-line treatment showed poor results, with a 5-year survival rate of only up to 10% and a median overall survival (OS) of <12 months.[4] Therefore, more effective treatment options are needed.
Angiogenesis is one of the main mechanisms that contribute to tumorigenesis, proliferation and metastasis, migration, and nutrient supply. Previously, anti-angiogenic therapy was given for various types of cancer, including breast, lung, colon, and hepatic cancers. Currently, molecular target therapy has become one of the effective treatment options for mGC.
The vascular endothelial growth factor (VEGF) family, which binds with VEGF receptors (VEGFR) and stimulates angiogenesis signaling, is among the key regulators of angiogenesis. The monoclonal antibody ramucirumab and the tyrosine kinase inhibitor apatinib have high selectivity for binding with and strongly inhibit VEGFR-2, thus decreasing VEGF-mediated tumor growth and metastasis. The results of phase II-III trials have confirmed that apatinib significantly improved OS and progression-free survival (PFS) with an acceptable safety profile in at least 2 lines of chemotherapy-refractory advanced gastric cancer compared with placebo.[5,6]
However, the clinical efficacy of antiangiogenic drugs combined with chemotherapy versus chemotherapy alone remains controversial. Previous studies have reported positive results on the OS benefits of monoclonal antibody ramucirumab combined with paclitaxel versus paclitaxel alone for gastric cancer after failure of first-line treatment.[7–9]
This study aimed to assess the efficacy and safety of apatinib plus chemotherapy compared with chemotherapy alone as second-line or later therapy in patients with advanced gastric cancer or mGC.
2. Methods
2.1. Patient selection
Patients with histologically confirmed mGC at Jiangsu Cancer Hospital & Research Institute were prospectively recruited in a 2-arm cohort clinical study. Patients who met the eligibility criteria were non-randomly assigned into 2 groups at a 2:1 ratio to receive either apatinib plus chemotherapy or chemotherapy alone.
The inclusion criteria were as follows:
-
(1)
pathologically confirmed advanced gastric or gastroesophageal junction adenocarcinoma (advanced disease was defined as primary tumor or local recurrence not eligible for complete surgical resection or the presence of metastatic disease);
-
(2)
history of failure of at least 1 line of chemotherapy;
-
(3)
indispensable 1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria as determined via computed tomography (CT) or magnetic resonance imaging (MRI);
-
(4)
age 18 to 75 years old;
-
(5)
Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0 to 1; and
-
(6)
a life expectancy of no less than 3 months.
The exclusion criteria were as follows:
-
(1)
uncontrolled blood pressure (140/90 mmHg or higher);
-
(2)
with bleeding tendency or receiving hemostatic drugs or anticoagulants;
-
(3)
with cardiac, hematologic, hepatic, and renal function incompatible for chemotherapy;
-
(4)
with health conditions influencing oral administration, for example, inability to swallow, chronic diarrhea, and intestinal obstruction;
-
(5)
with gastrointestinal bleeding tendency, for example, active ulcer lesions, stool occult blood (++), history of melena, and hematemesis, in the past 2 months; and
-
(6)
with any other condition that would make the treatment unsafe.
The trial was approved by institutional review board and the ethics committee by Jiangsu Cancer Hospital and all patients signed a written informed consent before participation. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, and Chinese law.
2.2. Treatment regimen
Apatinib was administered at a dose of 500 mg once a day for a 28-day cycle. The daily dosage could be decreased to 250 mg if patients experienced grade 3/4 adverse events (AEs). Patients in both groups received one of the following chemotherapy regimens:
-
(1)
modified fluorouracil (FU)-based regimen: FU 500 mg/m2 on days 1 to 5 or TS-1 50 mg bid on days 1 to 14 combined with platinum oxaliplatin 85 mg/m2 on day 1 or cisplatin 25 mg/m2 on days 1 to 3;
-
(2)
paclitaxel-based regimen: paclitaxel 135 mg/m2 or docetaxel (DOC) 75 mg/m2 on day 1 combined with a platinum chemotherapeutic;
-
(3)
FU, PTX/DOC, platinum;
-
(4)
irinotecan-based regimen: irinotecan 65 mg/m2 on days 1 and 8 combined with a platinum chemotherapeutic.
All regimens were repeated every 21 to 28 days for 2 to 6 cycles. Treatment interruption due to intolerance to persistent AE was allowed below 14 days (either continuously or cumulatively). Dose escalation was not permitted. Treatment was discontinued due to disease progression, intolerable of toxicity, or other personal reasons. (Fig. 1)
Figure 1.
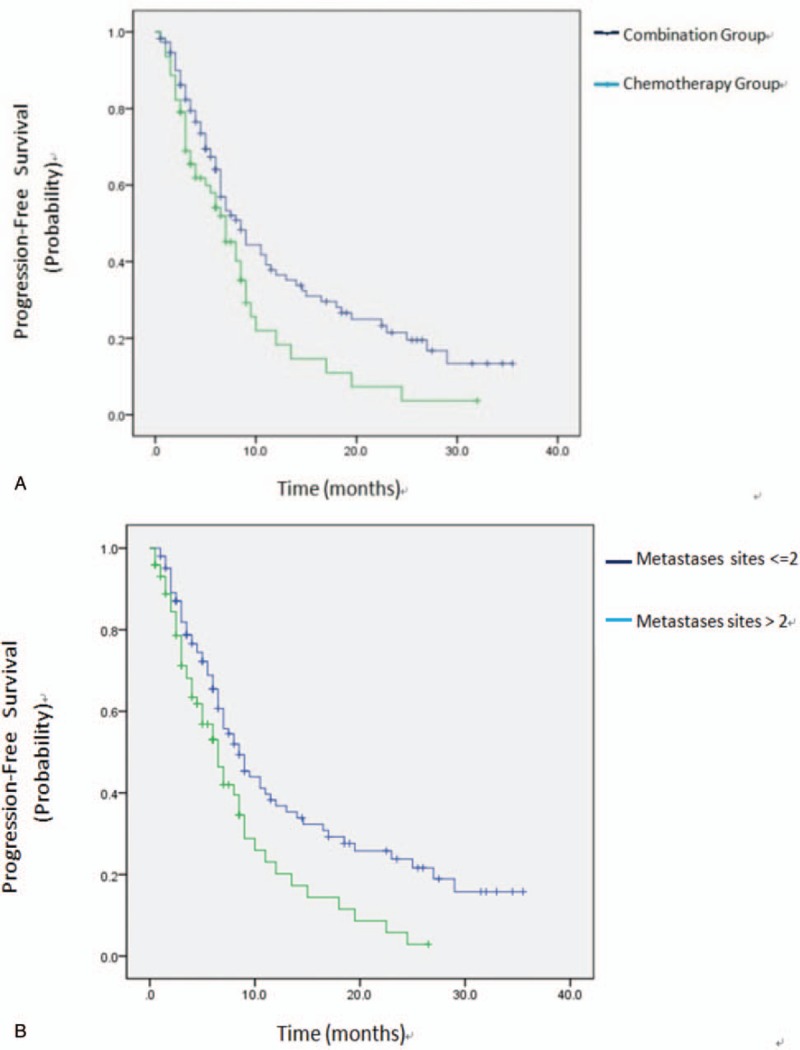
Kaplan–Meier estimates of PFS. (A) The median PFS was 8.5 months in the combination group as compared with 7.0 months in the chemotherapy group (P = .021). (B) The median PFS was 8.5 months in patients with ≤2 metastases sites as compared with 6.5 months in patients with >2 metastases sites (P = .005). PFS = progression-free survival.
2.3. Efficacy and safety
The primary end point was PFS, while the secondary end points were objective response rate (ORR), disease control rate (DCR), and toxicity. PFS was defined as the time from initiation of apatinib to disease progression. Treatment response was evaluated by investigators and independent radiologists as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the RECIST 1.1 criteria. ORR and DCR were computed as the sum of CR and PR and the sum of CR, PR, and SD, respectively. Treatment response was evaluated every 2 cycles, and follow-up CT and/or MRI findings were compared with baseline until disease progression. All AEs were evaluated using the National Cancer Institute Common Terminology Criteria for AEs version 3.0.[10]
2.4. Statistical analysis
Comparison of quantitative variables between groups was performed using t test. Pearson χ2 test was used to determine the association between categorical variables. Survival curves for PFS and corresponding 95% confidence intervals (CIs) were estimated via Kaplan–Meier method. The hazard ratios (HRs) and 95% CIs were estimated using the Cox's proportional hazards regression model. Potential factors to predict the PFS of apatinib were analyzed via univariate and multivariate analyses. Univariate analyses were performed using log-rank test, while multivariate analyses were performed using Cox's regression model based on results of the univariate analyses. All statistical analyses were 2-sided. The form of frequency counts and percentages were used to aggregate responses and AEs. All statistical analyses were 2-sided. Data analysis was performed using SPSS (version 21; IBM, Armonk, NY), and P <.05 was considered significant.
Before investigation, the total type I error (α) was set to 0.05, the power of test (1-β) was 80%, the enrollment period to 24 months, and the entire study period was 36 months. The required sample size was estimated to be 180 patients.
3. Results
3.1. Patient characteristics in the 2 groups
A total of 179 patients with mGC who had progressed or relapsed after undergoing at least 1 line of systemic therapy at Jiangsu Cancer Hospital &Research Institute between November 2014 and December 2016 were included. Of these, 115 were categorized to the combination group and 64 to the chemotherapy group. The median follow-up time was 32.6 months. Two patients in each group dropped off the study prior to first assessment due to clinical progression, toxicity, or personal reasons. In the end, 175 patients were analyzed (113 in the combination group and 62 in the chemotherapy group).
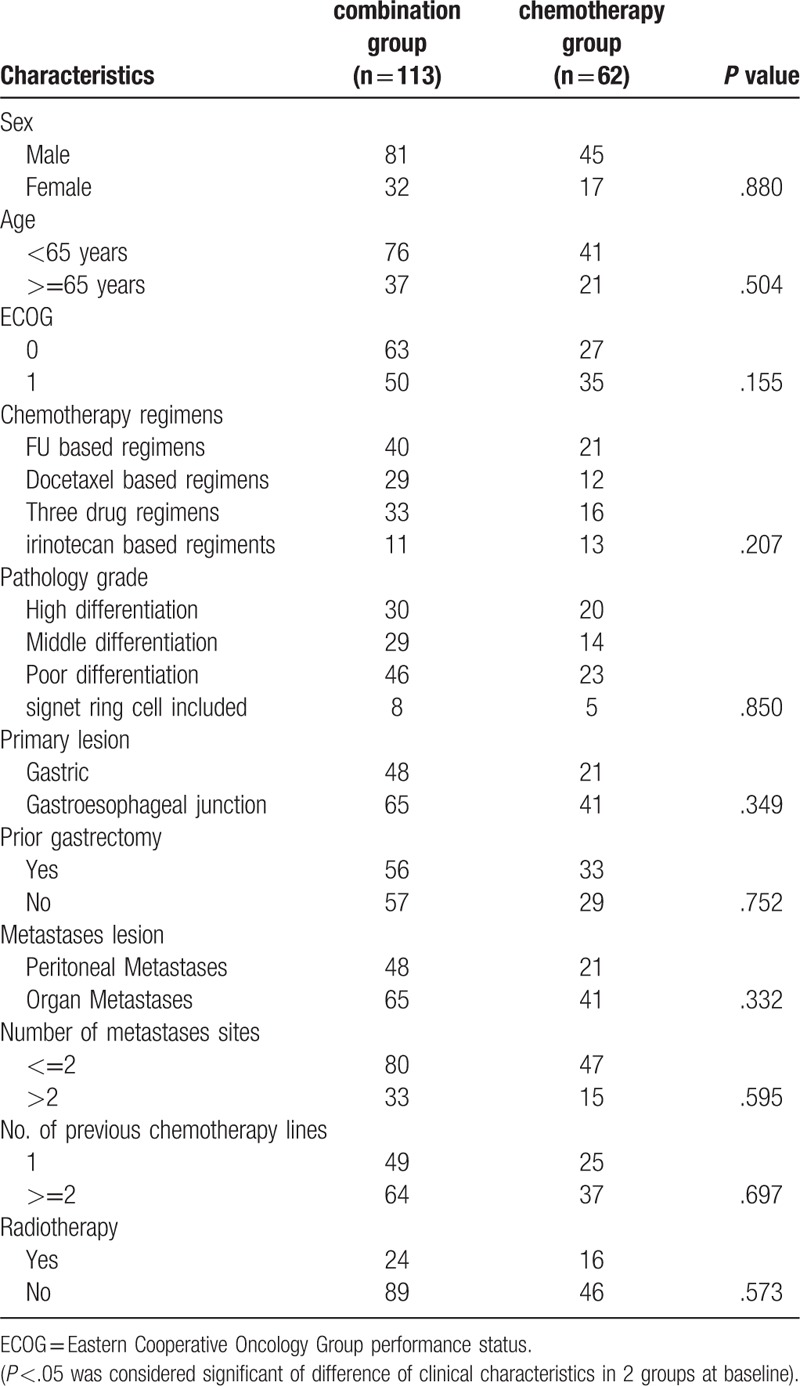
The baseline characteristics of the 2 groups were well balanced (Table 1). The median age in the combination group was 61.0 (range, 29–81) years, while it was 60.5 (range, 29–81) years in the chemotherapy group. The percentage of patients with an ECOG-PS of 0 in the combination group was relatively higher than that in the chemotherapy group (55.7% vs 43.5%), but the difference was not significant (P = .554). The percentage of patients who underwent complete surgical excision of primary disease (49.5% (56/113) vs 53.2% (33/62)) and received postsurgical radiotherapy (21.2% (24/113) vs 25.8% (16/62)) was lower in the combination group. Meanwhile, 26.8% (33/113) of patients in the combination group had >2 metastasis sites, while it was only 24.2% (15/62) in the chemotherapy group. A total of 770 cycles of chemotherapy were administered, and the mean number of chemotherapy cycles was not significantly different between the combination and chemotherapy groups (4.31 vs 4.56; P = .553).
Table 1.
Clinical characteristics of 2 groups at baseline (Pearson χ2 test).
3.2. Survival analysis of the 2 groups
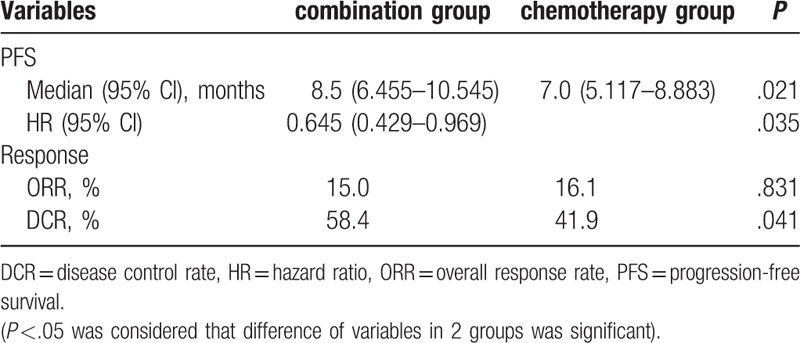
PD occurred in 72 (63.7%) patients in the combination group and in 44 (70.9%) in the chemotherapy group. The ORR of the combination group was 15.0%, while it was 16.1% in the chemotherapy group (P = .831). DCR was higher in the combination group than that in the chemotherapy group, and the difference was significant (58.4% vs 41.9%, P = .041; Table 2). Kaplan–Meier analysis demonstrated a significant improvement in PFS in the combination group (8.5 months, 95% CI: 6.45–10.54) as compared with that in the chemotherapy group (7.0 months, 95% CI, 5.12–8.88, P = .021), and the HR was 0.645 (95% CI, 0.429–0.969, P = .035).
Table 2.
Analysis of efficacy in 2 groups (Pearson χ2 test and Kaplan–Meier method).
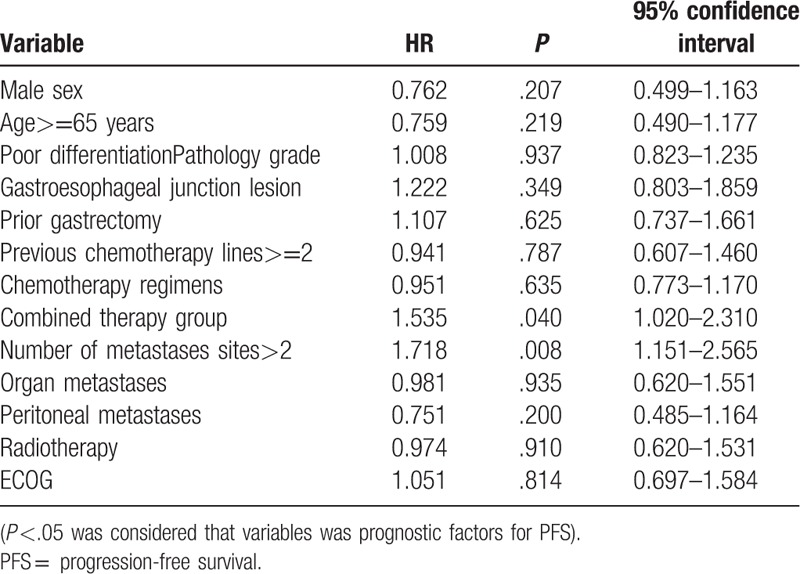
3.3. Univariate and multivariate analysis of prognostic factors with PFS
Univariate analysis showed that therapy group, number of metastases sites were prognostic factors for PFS (Table 3). Multivariate analysis with adjustment for confounding factors in the Cox regression model showed that combined therapy regimens (P = .040) and less than 2 of metastatic sites (P = .008) were independent prognostic risk factors and the risk factors for disease progression (Table 4).
Table 3.
Univariate analysis of independent prognostic factors (COX regression model).
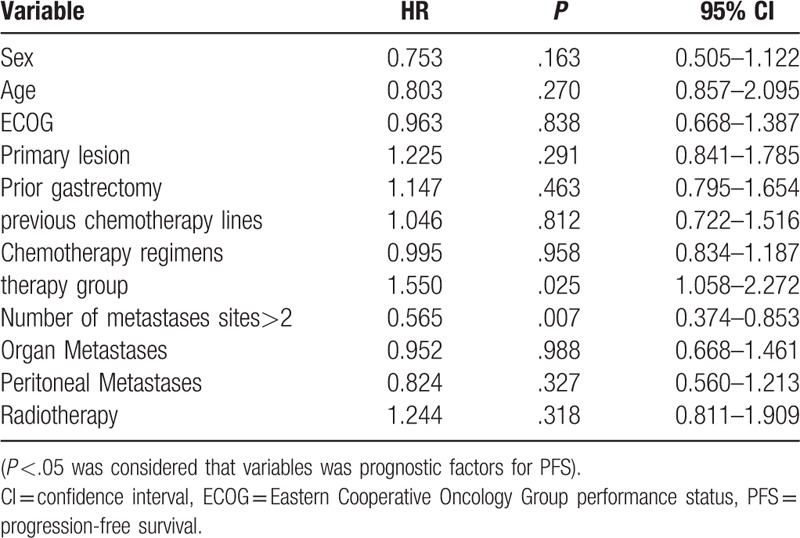
Table 4.
Multivariate analysis of independent prognostic factors (COX regression model).
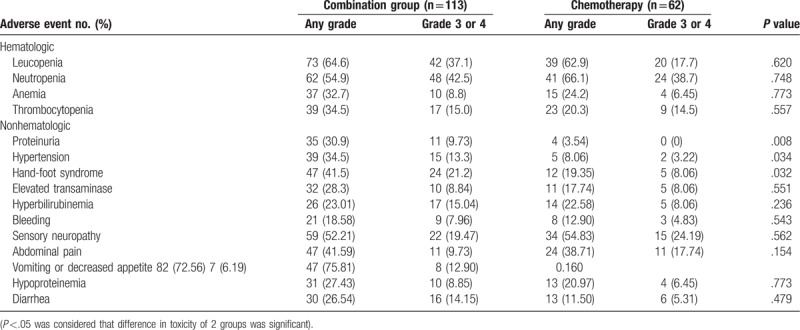
3.4. Safety analysis of the 2 groups
The safety analysis included a total of 175 patients who had received at least 2 cycles of therapy (Table 5). All toxicities were generally well tolerated. As compared with the chemotherapy group, dose modifications resulting from toxicity were more common in the combination group (13.8% v 6.4%); the most common of which were hand-foot skin reaction (HFSR), proteinuria, and hypertension. The type and incidences of the AEs are summarized in Table 5. Grade 3 to 4 HFSR (21.2% vs 8.6%; P = .0032), hypertension (13.3% vs 3.22%; P = .0034), and proteinuria (9.73% vs 0%; P = .008) were more common in the combination group than that in the chemotherapy group. The incidence of Grade 3/4 neutropenia (42.5% vs 38.7%; P = .748) and elevated transaminase (8.84% vs 8.06%; P = .551) was also higher in the combination group but the difference was not significant.
Table 5.
Analysis of safety in 2 groups (Pearson χ2 test).
4. Discussion
To our knowledge, this is the first prospective study demonstrating the efficacy of combined chemotherapy with or without apatinib for patients with at least first-line-failure mGC with an acceptable safety profile. The combination group met the primary end point by extending PFS for 1.5 months compared with chemotherapy group with an HR of 0.645. And we also demonstrated the improvements of DCR with statistical significance.
Gastric and gastroesophageal junction adenocarcinoma is the most common malignancy in East Asia and has high mortality rates owing to their high risk of recurrence and metastasis. First- to second-line chemotherapy often have poor efficacy and yield high toxicity, and patients often fail rescue treatment. Molecular-targeted has addressed the issue on lack of effective treatments for these malignancies. VEGF binds to VEGFR and triggers the dimerization and transphosphorylation of the intracellular tyrosine kinase domains. This causes cellular proliferation and endothelial cell survival and plays an important role in tumorigenesis and metastasis. Blocking angiogenesis pathways via specific inhibitors may lead to inhibition of malignant cell proliferation and differentiation.[11] VEGFR-2 antagonists are primarily linked to angiogenesis stimulation. Prolonged exposure to VEGF inhibitors could delay tumor growth and even maintain tumor regression. Anti-angiogenic blockers have been found to be effective in several tumors.[21] However, because of its poor efficacy when used alone, antiangiogenic therapies, regardless of type, are usually combined with chemotherapy, but the efficacy of combined treatment compared with chemotherapy alone is controversial. A meta-analysis showed a statistically significant improvement in OS and PFS via inhibition of VEGFR-2 pathways in patients with mGC.[12] The REGARD and RAINBOW trial[7,13] showed a significantly prolonged OS among patients treated with the monoclonal antibody ramucirumab combined with paclitaxel or placebo compared to those in the control group. Meanwhile, contrasting results were obtained in a small sample trial of ramucirumab combined with FOLFOX4.[8,9]
The tyrosine kinase inhibitor apatinib (Hengrui Pharmaceutical Co. Ltd) has been shown to significantly improve OS and PFS with an acceptable safety profile in patients with mGC refractory to 2 or more lines of prior chemotherapy. Apatinib has been proven to be safe and effective as third-line treatment for patients with advanced gastric cancer in phase II and III trials.[5,6] A small-sample retrospective study has also reported showed positive results about the therapeutic effect of apatinib as second-line treatment.[13] This prospective clinical trial aimed to evaluate the efficacy and safety of apatinib combined with chemotherapy after failure of at least first-line treatment. Our results showed that there was statistically significant trend toward improved PFS and good safety profiles in the combined group compared with that in the chemotherapy group. These findings are consistent with those reported in a prospective randomized controlled clinical study that combined apatinib and DOC for second-line therapy.[14,15] In this study, PFS but not OS was analyzed as the primary end point in this study because follow-up time was limited, and measuring OS was not possible. Meanwhile,2 studies concluded that PFS was strongly correlated with OS at 0.84 among patients mGC who underwent multi-line chemotherapy.[16,17] So we can concluded that combination of apatinib and chemotherapy could improve survival of mGC according to statistical difference of PFS in 2 groups.
No indicator for the efficacy of apatinib as a VEGFR-2 inhibitor has been identified. A retrospective study showed that early presence of anti-angiogenesis-related AEs including hypertension, proteinuria, or hand and foot syndrome during the first cycle of apatinib treatment was a viable biomarker of antitumor efficacy in patients with metastatic GC.[18,19] A study of patients with breast cancer showed that both hypertension and high expression of phosphorylated VEGFR-2 could serve as potential biomarkers for its treatment efficacy.[20] In this study, we found that combined therapy and metastatic sites <2 were independent prognostic factors of advanced gastric cancer, which is in accordance with the results of clinical trials on apatinib monotherapy.[5,6]
In conclusion, this prospective study showed that apatinib, a small-molecule VEGFR-2 inhibitor, combined with FU-, platinum-, paclitaxel-, or irinotecan-based chemotherapy is associated with prolonged PFS and increased DCR as compared with chemotherapy alone, in patients with gastric or gastroesophageal junction adenocarcinoma refractory to at least first-line chemotherapy, with manageable and tolerable side effects. The ongoing follow-up will provide more clinical data about the OS and AEs of apatinib and chemotherapy combination regimens in order to guide clinical practice. The results of this trial demonstrated that apatinib combined with chemotherapy could be a new treatment option for patients with mGC who progressed after 1 or more line of chemotherapy.
Acknowledgments
The authors thank all of the individuals who assisted with this study, especially the help in statistics from the Department of Epidemiology and Biostatistics at the School of Public Health, Nanjing Medical University, China.
Author contributions
Conceptualization: Xin-En Huang.
Formal analysis: YeSong GUO.
Methodology: JinHai Tang.
Project administration: JIE CAO.
Software: Xin-En Huang.
Footnotes
Abbreviations: AEs = adverse events, CI = confidence interval, CR = complete response, CT = computed tomography, DCR = disease control rate, DOC = docetaxel, FU = fluorouracil, HFSR = hand-foot skin reaction, HRs = hazard ratios, mGC = metastatic gastroesophageal junction adenocarcinoma, MRI = magnetic resonance imaging, ORR = objective response rate, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease, VEGF = vascular endothelial growth factor, VEGFR-2 = vascular endothelial growth factor receptor 2.
The authors declare that they have no conflicts of interest.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Thuss-Patience PC, Hofheinz RD, Arnold D, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann Oncol 2012;23:2827–34. [DOI] [PubMed] [Google Scholar]
- [3].Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–8. [DOI] [PubMed] [Google Scholar]
- [4].Hawkes E, Okines AF, Papamichael D, et al. Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur J Cancer 2011;47:1146–51. [DOI] [PubMed] [Google Scholar]
- [5].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebocontrolled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Qin S, Xu J, et al. Randomized, double-blind,placebo-controlled phase III trial of apatinib in patients with chemotherapyrefractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [7].Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a doubleblind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. [DOI] [PubMed] [Google Scholar]
- [8].Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): randomized, double-blind, multicenter phase 2 trial. J Clin Oncol 2014;32: [Google Scholar]
- [9].Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter Phase II trial. Ann Oncol 2016;27:2196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].An MW, Dong X, Meyers J, et al. Evaluating continuous tumor measurement-based metrics as phase II endpoints for predicting overall survival. J Natl Cancer Inst 2015;107: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Casak SJ, Fashoyin-Aje I, Lemery SJ, et al. FDA approval summary: ramucirumab for gastric cancer. Clin Cancer Res 2015;21:3372–6. [DOI] [PubMed] [Google Scholar]
- [12].Giandomenico Rovielloa,b,c, Karol Polomd, et al. Targeting VEGFR-2 in Metastatic Gastric Cancer: Results Froma Literature-Based Meta-Analysis. Cancer Investigation 2017; Vol.35,No.3,187–194. [DOI] [PubMed] [Google Scholar]
- [13].Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Y, Gou M, Han C, et al. Efficacy and safety of apatinib as second-line therapy for advanced gastric cancer: a single-center observational study. Anticancer Drugs 2017. [DOI] [PubMed] [Google Scholar]
- [15].Wang Z, Dai G, et al. Apatinib combined with docetaxel in second-line treatment of advanced gastric cancer: a prospective randomized controlled clinical study. Annal Oncol 2017;28: [Google Scholar]
- [16].Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget 2017;8:29346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu L, Yu H, Huang L, et al. Progression-free survival as a surrogate endpoint for overall survival in patients with third-line or later-line chemotherapy for advanced gastric cancer. OncoTarget Ther 2015;8:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–91. [DOI] [PubMed] [Google Scholar]
- [19].Liu Z, Qin Z, Wang Z, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol 2017;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141–51. [DOI] [PubMed] [Google Scholar]
- [21].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumour growth and angiogenesis. J Clin Oncol 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- [22].Kang EJ, Im SA, Oh DY, et al. Rinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum,and taxane in gastric cancer: Treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013;16:581–9. [DOI] [PubMed] [Google Scholar]


