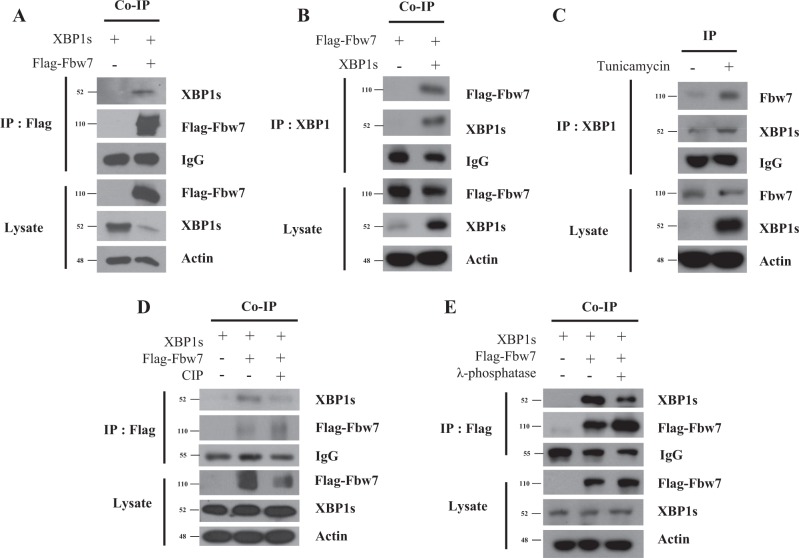
Fig. 1. Fbw7 interacts with XBP1s in phosphorylation-dependent manner.
a Co-immunoprecipitation (Co-IP) of transiently expressed XBP1s and FLAG-Fbw7. HEK293FT cells were co-transfected with XBP1s and FLAG-Fbw7. Immunoprecipitation was detected with anti-FLAG. b Co-immunoprecipitation (Co-IP) of transiently expressed XBP1s and FLAG-Fbw7. HEK293FT cells were co-transfected with XBP1s and FLAG-Fbw7. Immunoprecipitation was detected with anti-XBP1. c Immunoprecipitation of tunicamycin induced endogenous XBP1s and Fbw7. HEK293FT cells were treated 1 μg/ml tunicamycin 24 h. Immunoprecipitation was detected with anti-XBP1. d XBP1s interacts with Fbw7 in a phosphorylation-dependent manner. Transient expressing XBP1s and/or FLAG-Fbw7 lysates were treated with calf intestinal alkaline phosphatase (CIP), and subjected to co-immunoprecipitation. e Transient expressing XBP1 and/or FLAG-Fbw7 lysates were treated with λ-phosphatase, and subjected to co-immunoprecipitation. All western blot analyses were carried out with anti-XBP1and anti-FLAG antibodies. Actin was used for an internal control