Abstract
The frequency of extreme drought and heavy rain events during the vegetation period will increase in Central Europe according to future climate change scenarios, which will affect the functioning of terrestrial ecosystems in multiple ways. In this study, we simulated an extreme drought event (40 days) at two different vegetation periods (spring and summer) to investigate season-related effects of drought and subsequent rewetting on nitrifiers and denitrifiers in a grassland soil. Abundance of the microbial groups of interest was assessed by quantification of functional genes (amoA, nirS/nirK and nosZ) via quantitative real-time PCR. Additionally, the diversity of ammonia-oxidizing archaea was determined based on fingerprinting of the archaeal amoA gene. Overall, the different time points of simulated drought and rewetting strongly influenced the obtained response pattern of microbial communities involved in N turnover as well as soil ammonium and nitrate dynamics. In spring, gene abundance of nirS was irreversible reduced after drought whereas nirK and nosZ remained unaffected. Furthermore, community composition of ammonia-oxidizing archaea was altered by subsequent rewetting although amoA gene abundance remained constant. In contrast, no drought/rewetting effects on functional gene abundance or diversity pattern of nitrifying archaea were observed in summer. Our results showed (I) high seasonal dependency of microbial community responses to extreme events, indicating a strong influence of plant-derived factors like vegetation stage and plant community composition and consequently close plant-microbe interactions and (II) remarkable resistance and/or resilience of functional microbial groups involved in nitrogen cycling to extreme weather events what might indicate that microbes in a silty soil are better adapted to stress situations as expected.
Introduction
The IPCC report of 20141 has indicated major changes related to climate in the northern hemisphere, especially in the precipitation variability, for the next 20 years. Major scenarios predict an increase in the frequency of long lasting drought periods, serious flooding or even both following each other1–4. These extreme fluctuations of water availability may lead to a reduction of the soil quality as well as the ecosystem benefits provided by soils which includes plant production or carbon sequestration. In this respect, the performance of microbes in soil and changes in both structure and function of the soil microbiome in response to shifts in the climatic conditions play a very important role as bacteria, fungi and archaea can be considered as the architects of the soil quality and catalyze most nutrient turnover processes5.
Several studies have shown that drought and decreased water potentials in soil act as severe stress factors for microbes6–8. The mobility of microbes as well as the substrate diffusion is reduced and therefore nutrient resources for microbes are limited under drought conditions9. As a consequence, most biogeochemical turnover processes are slowed down or stopped7,8. However, consequences of different drought time points during the vegetation period on the observed response pattern of the soil microbiome are not well understood. Waldrop and Firestone10 reported that the composition of microbial community shows seasonal changes and that these patterns are largely influenced by seasonal plant community effects and also by seasonal differences in soil moisture. Furthermore, Bardgett et al.11 mentioned an increasing amount of studies dealing with seasonal changes in root exudates and resulting correlations with belowground properties and plant nutrient supply, nutrient cycling and growth. These seasonal dynamics are important as they control the nutrient availability and interactions between plants and microbes.
We hypothesize that microbial response pattern seasonally differs to a large extent after rewetting due to seasonal changes in plant biomass, quality and amount of root exudates, turnover of organic matter and litter decomposition. In order to test this hypothesis, we performed a plot experiment in grassland soils with simulated extreme droughts followed by intensive rewetting. The drought was set either in May - June (D1) or July - August (D2). The microbial response pattern and the chemical soil properties were measured at the end of the drought period as well as one and two and four weeks after the rewetting event. For comparison, control plots were set up that did not undergo the drought/rewetting cycle. Ammonia oxidizing and denitrifying microbes were selected for the study in order to compare microbial groups with different physiologies. Ammonia oxidizers are autotrophs and catalyze the first step of nitrification (the oxidation of ammonia to nitrite, whereas denitrifiers are typical heterotrophic microbes which use nitrate or other forms of oxidized nitrogen as alternative electron acceptor in the absence of oxygen12. Both groups of microbes also differ in their diversity: ammonia oxidation can only be performed by a very small group of specialized bacteria and archaea (AOB and AOA) or by the recently discovered comammox bacteria belonging to the genus Nitrospira13, whereas in contrast many microbes in soil are capable for denitrification and phylogenetically widespread14. In this study, the abundance as well as the diversity of the microbial groups of interest were assessed using quantitative real-time PCR and terminal restriction fragment length polymorphism (t-RFLP) using marker genes typically for ammonia oxidizers or denitrifiers (amoA, nirS/nirK and nosZ).
Results
Soil water content
Before the simulation of the spring drought started in May, the soil moisture content of C and D1 plots was comparable (38 vol. %) which is equivalent to 95% of maximal water holding capacity (WHCmax) (Fig. S1). During D1 the soil moisture constantly decreased to a minimum of 16 vol. % (40% WHCmax), at the last day of the drought (Table 1), with a significant difference over time (p = 0.008), whereas in the control plots only a slight decrease due to the natural precipitation levels were observed. Within one week after rewetting, the soil moisture levels of D1 plots and C plots were comparable (27 vol. %; t1). A similar trend was observed during summer drought. At the end of June (before starting the drought simulation), the soil moisture of D2 and C plots was comparable (24 vol. %; 60% WHCmax; Fig. S1). The simulated drought resulted in reduced soil moisture in D2 plots at the end of the drought period (13 vol. %; t0) and differed significantly (p = 0.002) from the control plots. The rewetting of D2 plots resulted in a significant increase (p = 0.000) of soil moisture levels (33 vol. %; 38% WHCmax; t1) which was comparable to C plots (30 vol. %; t1).
Table 1.
Soil moisture (SM) [vol%], Soil chemical properties [µg g−1 dw] and number of terminal restriction fragments (TRFs) of the archaeal amoA AOA gene for drought treatment and control in spring (D1 and C) and summer (D2 and C) over the sampling period.
| Time | Treatment | SM | NH4+-N | NO3−-N | TRFs | |
|---|---|---|---|---|---|---|
| spring | 29th June | C | 26.29 (12.01)A | 2.05 (0.55)A | 1.71 (1.47)A | 52 (18)A |
| (t0) | D1 | 16.02 (0.92)a | 1.61 (0.23)a | 0.74 (0.67)a | 66 (8)a | |
| 07th July | C | 28.79 (10.75)A | 1.42 (0.26)B | 2.88 (1.75)A | 56 (10)A | |
| (t1) | D1 | 27.85 (7.78)b | 1.12 (0.29)b | 2.75 (1.30)bc | 68 (15)a | |
| 14th July | C | 28.87 (7.30)A | 0.77 (0.02)C | 2.52 (0.82)A | 54 (6)A | |
| (t2) | D1 | 26.71 (7.83)a | 0.76 (0.18)b | 2.71 (0.53)b | 59 (6)a | |
| 28th July | C | 37.89 (5.42)A | 1.42 (0.20)*B | 3.79 (1.03)A | 53 (20)A | |
| (t3) | D1 | 29.82 (8.00) b | 1.03 (0.25)*b | 4.30 (0.80)c | 54 (3)a | |
| summer | 10th August | C | 26.74 (3.48)*AB | 0.98 (0.21)A | 2.95 (1.34)A | 51 (10)A |
| (t0) | D2 | 13.24 (6.54)*a | 0.81 (0.33)a | 3.92 (1.99)ab | 61 (13)a | |
| 18th August | C | 29.77 (5.25)B | 1.09 (0.24)A | 3.67 (2.58)A | 60 (8)AB | |
| (t1) | D2 | 33.17 (4.09)b | 1.00 (0.31)a | 2.80 (1.57)a | 63 (10)a | |
| 25th August | C | 21.31 (4.53)*A | 1.03 (0.09)A | 2.06 (0.90)*A | 73 (14)B | |
| (t2) | D2 | 27.74 (2.13)*b | 1.19 (0.16)a | 4.09 (1.35)*ab | 74 (10)a | |
| 08th September | C | 24.82 (4.18)AB | 1.06 (0.15)A | 3.44 (2.24)A | 63 (11)AB | |
| (t3) | D2 | 28.17 (3.77)b | 0.94 (0.08)a | 5.79 (1.33)b | 54 (12)a |
Standard deviations are shown in brackets. Significant differences (p < 0.05) between C and D1 or C and D2 are marked with asterisks and were calculated with a student’s t -test. Significant differences (p < 0.05) over time were calculated separately for C (capital letters), D1 and D2 (small letters) via repeated measurement ANOVA (Tukey-HSD) and are indicated by different letters.
Soil chemical parameters
Highest ammonium concentrations were measured at t0 (end of the drought period; Table 1) in D1 (1.61 µg g−1 dw) and C plots (2.05 µg g−1 dw) plots and decreased significantly after rewetting. In contrast, nitrate concentrations increased significantly after rewetting up to 4.30 µg g−1 dw (t3) in D1 but remained constant at control plots (Table 1). For both ammonium and nitrate concentrations no treatment effect was observed when comparing D1 and C plots, except a temporary higher ammonium concentration in D1 at t3. In contrast to the spring drought, ammonium and nitrate concentrations during the summer drought were not affected by time or treatment (except a temporary higher nitrate concentration at D2 compared to C plots at t2), ranging from 0.81 µg g−1 dw (t0) to 1.19 µg g−1 dw (t2) for ammonium and 2.06 µg g−1 dw (t2) to 5.79 µg g−1 dw (t3) for nitrate.
Abundance of ammonia-oxidizers and denitrifiers
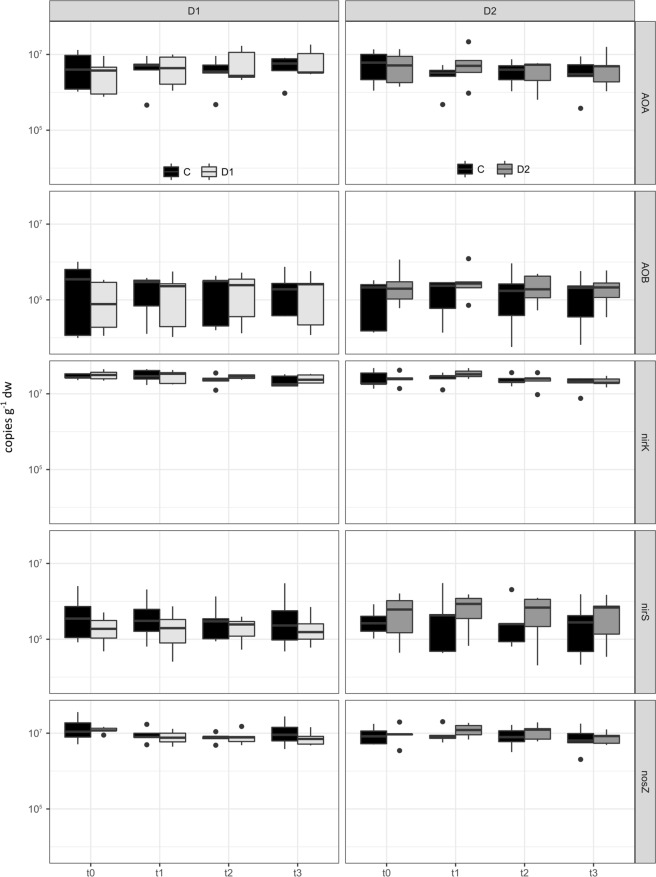
The abundance of archaeal ammonia-oxidizers (AOA), remained constant during the sampling period for both control and drought/rewetting treatment in spring and summer (3.08–7.55 × 106 copies g−1 dw; Fig. 1). Furthermore, the abundance of AOA was comparable between C and D plots. As expected, the number of bacterial ammonia-oxidizer (AOB) was approximately one order of magnitude lower compared to AOA and ranged between 1.47–4.04 × 105 copies g−1 dw (Fig. 1). As for AOA, no significant differences between treatments for both, control and drought/rewetting in spring and summer were observed for AOB.
Figure 1.
Gene abundance for archaeal and bacterial ammonia-oxidizers based on gene copy numbers of the amoA gene as well as for nitrite reducers harboring the nirK gene, nitrite reducers harboring the nirS gene and N2O reducers harboring the nosZ gene shown in gene copies g−1 dw. Left side shows spring drought event: bars for C (black) and D1 (light grey), right side shows summer drought event: bars for C (black) and D2 (dark grey). Sampling took place on the last day of the drought (t0), one (t1), two (t2) and four (t3) weeks after rewetting for D1 and D2, respectively.
For denitrifiers, the abundance of nirK harboring nitrite reducers was about two orders of magnitude higher compared to the nirS harboring counterpart (1.98–3.47 × 107 copies g−1 dw compared to 2.22–8.02 × 105 copies g−1 dw; Fig. 1) during the whole experimental period independently from the two drought simulation treatments. Whereas, abundance of nirK harboring denitrifiers remained constant during time and did neither respond to D1 nor D2 treatment compared to the respective controls, nirS harboring nitrite reducers were irreversible reduced after spring drought (p > 0.05). Similar to the denitrifiers harboring the nirK gene, neither seasonal nor treatment related effects were observed for denitrifiers harboring the nosZ gene (6.65 × 106–1.57 × 107 copies g−1 dw). Overall the abundance of nosZ harboring nitrous oxide reducers was significantly lower than the abundance of nitrite reducers (nirK + nirS).
Overall, similar results were obtained when abundance data of ammonia oxidizers was related to ng of extracted DNA (data not shown).
Diversity of ammonia-oxidizing archaea
The diversity analysis for the archaeal ammonia oxidizers based on the amoA gene revealed 104 different terminal restriction fragments (TRFs). Although not significant due to high replicate variability, a trend for higher TRF richness was observed for D1 (54–68 TRFs) compared to C (52–56 TRFs) plots (Table 1). AOA community profile in spring was dominated by four TRFs (142, 162, 253 and 274), accounting for 41–65% of total peak height (Fig. 2a). Three of them (TRF 142, 253 and 274) were positively affected by drought, showing a higher mean relative abundance at D1 compared to controls (p > 0.05). While TRFs 253 and 274 remained constant after rewetting, TRF 142 decreased significantly from 17 to 5% of total peak height at t3. In contrast, TRF 162 increased significantly after rewetting from 9 to 36% (t3). In silico analysis using amoA_AOA Feifei-Liu reference database of FunGene revealed that TRF 162 is most likely assigned to different members of Thaumarchaeota including Candidatus Nitrososphaera evergladensis, Candidatus Nitrosophaera viennensis, Candidatus Nitrosotalea devanaterra and Candidatus_Nitrosoarchaeum_limnia_SFB1) while TRF 253 might be assigned to Nitrosopumilus maritimus (group 1.1a) and Candidatus Nitrosotenuis. The same four fragments (142, 162, 253 and 274) dominated AOA terminal restriction length polymorphism (T-RFLP) profiles in summer, accounting for 52–67% of total peak height (Fig. 2b). In contrast to spring, no influence of drought and rewetting could be observed at D2.
Figure 2.
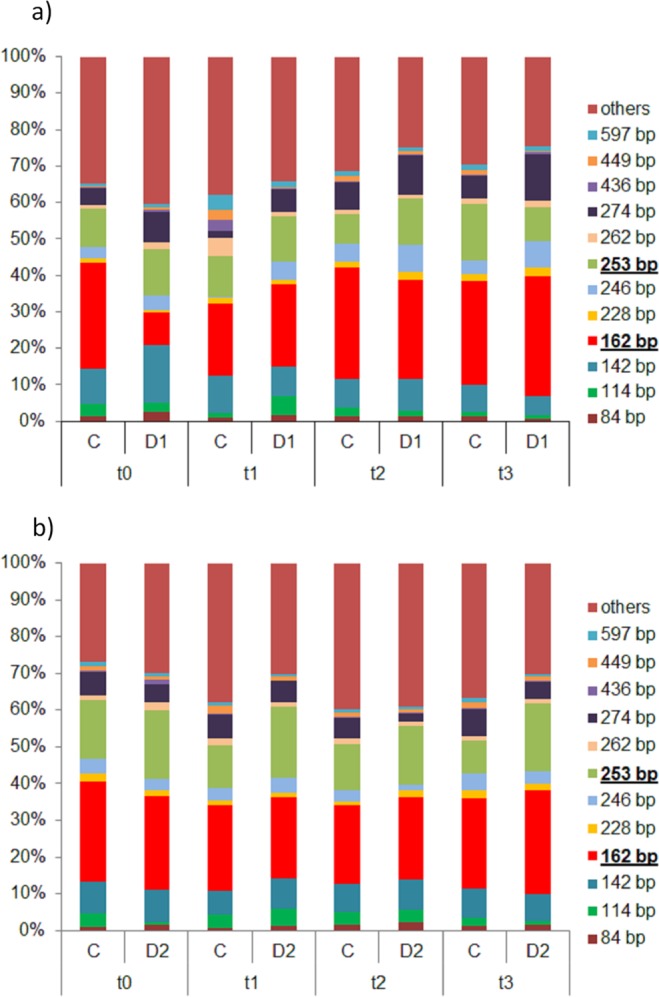
Relative abundance of the TRFs of the amoA AOA gene in percent [%] for (a) spring drought event (D1 and C) and (b) summer drought event (D2 and C). TRFs smaller than 3% are combined as “others”. Sampling took place on the last day of the drought (t0) and one (t1), two (t2) and four (t3) weeks after rewetting for D1 and D2, respectively. Most dominant peaks (162 and 253) are marked in the legend with bold and underlined letters.
Discussion
Effects of the drought simulations
Water contents in response to D1 and D2 treatments were highly reduced at the end of both drought periods, whereas, in the control plots the soil moisture contents were above 60% maximal water holding capacity (WHCmax) at the same time points. Taking into account that a soil water content of around 60% WHCmax presents the best conditions for microbial activity in terms of oxygen- and water availability as well as nutrient distribution in upland soils15, the spring as well as the summer drought simulation in this study might have resulted in a potential improvement of the living conditions for oxidative and a potential worsening for reductive soil microbes. Considering that denitrification is a process to gain alternative electron acceptors under anoxic conditions, denitrifiers should be negatively affected by simulated drought events. Interestingly, we observed a reduced abundance of denitrifiers harboring the nirS gene but not of those caring the nirK and nosZ gene after spring drought (D1) compared to the control plots, suggesting a higher drought sensitivity for nirS harboring microorganisms. This is in accordance with Hartmann et al.16, showing that drought in pasture soils did not affect nirK and nosZ harboring microbes but decreased abundance of denitrifiers caring the nirS gene. Moreover, as this negative effect on nirS harboring denitrifiers was only found after spring but not after summer drought our results imply that these drought effects were strongly driven by the season. This might be explained by seasonal differences due to plant growth stage or changes in root exudates as mentioned earlier by Kaiser et al., Rasche et al. and Regan et al.17–19. During early summer times (end of D1 treatment) plants have a higher uptake rate of nitrogen to meet their growth demands and to build up biomass compared to late summer times and consequently compete highly with microbes for nitrogen20,21. Hence, less nutrients are available for microbes that lead, amongst others, to a decrease in gene abundances. These seasonal variations affect for example microbial community composition of denitrifying bacteria22 and may explain differences in the dynamics of nirS harboring denitrifiers after spring and after summer drought. Although nosZ abundance was not affected by drought it has to be considered that in the present study only the “classical” nosZ gene (nosZI) was investigated. Recently, a new cluster of atypical nosZ genes, termed clade II nosZ genes, was discovered23 which encodes for enzymes catalyzing the same reaction but having <50% amino acid sequence similarity with type I NosZ enzymes. These nosZII sequences are often at least as abundant as nosZI in soil, suggesting that many N2O reducers were not covered in the present study. As clade II organisms were found to be more sensitive to environmental parameters24, potential drought effects might have been overlooked. However, nosZII gene abundance is positively linked to pH25. Thus, this clade might be of minor importance in the studied ecosystem due to highly acidic soil (pH 4.1). Given the minor drought effect observed on denitrifier gene abundance and the results of previous studies showing that changing moisture regimes had only small or even no effect on denitrifier community26,27, denitrification was not investigated in more detail (e.g. by community fingerprinting) in the present study.
Contrary to denitrification, drought might be expected to increase oxidative processes like nitrification. Surprisingly, our results showed no change in abundance of AOA and AOB after spring and summer drought compared to the respective controls. This might be explained by the low soil pH of 4.1, which shifts the equilibrium between ammonia and ammonium towards ammonium28 (ratio of NH3/NH4+ = 1:20,000 at pH 529 and p Ka for NH4+ ⇆ NH3 equilibrium is 9.2530), resulting in worse conditions for soil microbes preferring ammonia31,32. In addition, acid soils bind less positive loaded compounds like ammonium or ammonia33, what might be even more strengthened with drought. Further, low soil pH affects microbial activity more negative than plant activity34 and consequently favors plants acting as competitors for ammonium uptake with microbes12,35,36. This shift was found to be even more pronounced at simulated extreme drought events in grassland soil6. Although drought did not influence abundance of archaeal and bacterial ammonia oxidizers, this might not exclude shifts in diversity pattern. Coinciding with Gubry-Rangin et al.37, we observed that archaea dominated over bacteria in acid soil. Moreover, archaea were shown to be the main performer of nitrification in acidic soil38. Thus, we focused on community composition of AOA in our study. The predominant TRF 162 in all samples could be assigned (I) to a low pH adapted soil isolate Candidatus Nitrosotalea devanaterra, which has an growth optimum at pH 4.0–5.5 and is able to oxidize both ammonia and ammonium29 and (II) to different members of Candidatus Nitrososphaera, which are well adapted to environmental changes by formation of biofilms, detoxification and adhesion and thus dominates 15 out of 16 soils39. However, it has to be considered that assignment was based on in silico analysis using amoA_AOA reference database of FunGene without sequencing confirmation. Not only abundance but also community composition of ammonia oxidizing archaea remained unaffected by the drought simulations, which supports earlier observations on resistance of AOA towards drought stress40 and a good adaptation of AOA to a broad range of growing conditions and substrate concentrations41,42.
Effects of rewetting
Rewetting was expected to improve the availability of soil water for microbial communities and consequently increase microbial growth. Several studies described an increase in C and N mineralization within one to four days after rewetting of dry soils43–47, which indicates a release of labile substrates due to microbial cell lysis or osmoregulation. This additional input of ammonium, nitrate or nitrite due to degraded biomass after rewetting might be expected to result in higher abundance of nitrifiers and denitrifiers. However, this could not be observed in the present study. Possible explanations for missing the momentary peak resulting from the fast turnover rate of N mineralization could be implicated (I) by our sampling time points, (II) the quantification of genes (DNA level) instead of transcripts (RNA level) which might have been correlated stronger to the real microbial activity and (III) by a strong competition between plants and microbes for ammonium and nitrate, supporting data from Bannert et al.35. Besides the potential positive effect of rewetting due to higher substrate input, oxidative processes like nitrification might be negatively influenced due to decreased oxygen diffusion. However, a decrease in archaeal and bacterial ammonia oxidizers with increasing soil moisture as described by Horz et al.48 was not observed in the present study, probably since the water content was not exceeding 70% WHCmax after rewetting. Furthermore, AOA can also act as heterotrophic microbe and hence not only use ammonia but also organic N as N source31,49,50. Interestingly, diversity pattern of AOA changed significantly after rewetting, although gene abundance remained constant. While the predominant TRF 162 increased, TRFs 142 and 84 decreased with increased soil moisture, indicating that some archaeal ammonia oxidizers might be better adapted to changing environmental conditions than others. Coinciding with responses to simulated drought events, the observed changes were much more pronounced after spring drought rewetting compared to rewetting after summer drought, which supports our hypothesis that microbial responses to extreme events are strongly dependent on the season due to plant derived effects as plant biomass, plant community composition and the physiological state of grassland on the experimental plots differ between the setting of the spring respectively summer drought. This seasonal impact was also reported by Grant et al.51 as changes in functional composition of the grassland could have been shown for spring but not for summer treatment within the present experiment. A significant increase (p < 0.001) in the aboveground net primary production (ANPP) of forbs and a significant decrease (p = 0.038) in ANPP of grasses gave evidence that the composition of species was significantly affected by the timepoint of the drought treatment. This was furthermore, confirmed by the functional group evenness that was significantly impacted by D1. A shift in the competitive balance from dominant grasses to subdominant forbs could be reported. As grasses have a shallow root system concentrated in the upper soil layer they seem to be more vulnerable to drought stress as forbs with a deeper root system. This change of root depth and therefore changes in root exudate and its composition can furthermore impact microbial communities.
Conclusion
Our results indicate a strong seasonal dependency of microbial responses to extreme climatic events like drought and subsequent rewetting, which might be explained by plant derived effects, e.g. seasonally different plant development stage, plant community composition and, consequently, root exudate quantity and quality. However, further studies mainly simulating repeated drying-rewetting cycles are needed to draw conclusions for climatic modeling out of this dataset, as response pattern of microbes might differ after repeated stress simulation. Studies within a sandier soil texture, where water storage capacity is lower, should be done to compare and verify results related to weather extremes in different types of soil. In addition, this study was conducted with DNA, which serves as a proxy for the abundance of selected microbial groups; thus, further analysis with RNA can point out the expression of microbial genes that are involved in the metabolism of root exudates, soil processes and mineralization of nutrients. Moreover, additional genes should be analyzed to give a more detailed view of effects of changing soil water regimes on N cycling processes. Additionally, nitrification and denitrification assays should be performed to link genetic potential directly to enzyme activity. Overall, our two experiments indicate a high resilience and resistance to extreme weather events on the level of selected microbial groups linked to the nitrogen cycle that could be confirmed and can give evidence that microbes in a silty soil are better adapted to stress situations as expected.
Methods
Study site
The study was part of the EVENT 2 experiment at the Ecological-Botanical Garden of the University of Bayreuth, Germany (49°55′19″ N, 11°34′55″ E and 365 m above sea level)52,53. The plant community has been typical for semi-natural-grassland and was dominated by tall grasses, especially Alopecurus pratensis and Arrhenatherum elatius. The mean annual temperature was 8.2 °C and the mean annual precipitation of the region was 724 mm (1971–2000). The soil type has been classified as a Gleysol54. The experimental site was abandoned for 25 years before the experiment started without any plowing or addition of fertilizers. The A - horizon (0–30 cm depth) had a soil texture of 42% sand, 43% silt, 15% clay, a total carbon content of 2.34%, a total nitrogen content of 0.20%, a pH of 4.1 (1 M KCl), a water holding capacity of 40% and a permanent wilting point of 15%55.
Experimental design and sampling
In this experiment we simulated the following treatments: spring drought (D1) (19th May to 29th June 2009), summer drought (D2) (30th June to 10th August 2009) and control (C). The experiments were set up using a latin square design, with a size of 1.5 × 1.5 m per plot55. Each treatment was replicated five times, resulting in 15 plots in total.
Each drought period lasted 42 days based on the duration of a statistically calculated local 1,000-years extreme event and was realized by using rain-out shelters as described by Walter et al.53. Briefly, rain-out shelters, starting in a height of 0.8 m above soil, were used to exclude rain but to enable ventilation and to avoid overheating of the shelters. The shelters were made of a transparent polyethylene (PE) foil with a light-permeability of 90% for an optimal light perception. After each drought period the rain-out shelters were removed and compensation irrigations with tap water were added by simulating a heavy rainfall event (Fig. S1) using a portable irrigation system. According to the naturally occurring water amounts during each drought treatment 102.5 mm water was added to the plots after D1 treatment and 187.5 mm after D2 treatment. To prevent water-run-off by simulated heavy rainfall events, the irrigation was divided into two applications on one day. To avoid lateral surface water flow plastic sheet pilings were placed around each plot down to a depth of 0.2–0.25 m.
Samples were taken at the last day of the drought (t0) and one, two and four weeks after the simulated heavy rainfall event (t1, t2, and t3). At each sampling date, three soil samples were collected from each of the five replicate plots per treatment with a corer of 3-cm diameter and pooled (5–15 cm depth; 0–5 cm were discarded because of a high root content). After each sample soil, the corer was sterilized with 70% Ethanol to prevent cross-contamination. All samples were immediately frozen at −80 °C until further processing for molecular analyses or stored at 4 °C for analyses of soil chemical parameters.
Soil moisture
A continuous soil moisture measurement was performed every hour by using a frequency domain (FD)-sensors (ECH2O, Decagon devices, Pullman, USA) as described by Walter et al.53. Each logger was installed in each plot in undisturbed soil in a depth of −2 to −7 cm, respectively. The soil moisture data was calculated with means of each replicate per treatment (C, D1 and D2) and given in volume % [vol. %]. Additionally, soil moisture of sampled soils was determined by drying 2 g of fresh soil at 105 °C for 24 hours in an aluminum bowl and given per gram dry weight [g−1 dw].
Soil chemical parameters
In order to determine the content of ammonium (NH4+-N), and nitrate (NO3−-N), soil samples were extracted by shaking 5 g fresh soil with 20 mL of 1 M KCl for 30 min on a rotary shaker. The soil suspension was filtered using 0.45 µm pore-size filters (Whatman International LTD, VWR, Germany), diluted 1:100 using sterile MilliQ water and stored at −20 °C until further analyses. The contents of ammonium (NH4+-N) and nitrate (NO3−-N) were determined by a continuous flow analysis with a photometric autoanalyzer (CFA-SAN Plus, SkalarAnalytik, Germany)35.
DNA extraction
DNA was extracted from 0.5 g fresh soil using the FastDNA® SPIN Kit for Soil (MP Biomedicals, Canada) and the Precellys24 Instrument (Bertin Technologies, France) according to the manufacturer’s protocol. After extraction, DNA quantity and quality were determined using the spectrophotometer Nanodrop (PeqLab, Germany) (73–214 ng µL−1 DNA). Subsequently, DNA was stored at −20 °C until further processing.
Quantitative Real-Time PCR Assay
Quantitative Real-Time PCR (qPCR) for all marker genes used to describe ammonia oxidizers as well as denitrifiers was carried out on a 7300 Real-Time PCR System (Applied Biosystems, Germany) using SYBR green as fluorescent dye as previously described56. The PCR was performed in 96-well plates (Applied Biosystems, Germany): Details on marker genes and PCR conditions are described in Table 2. A dilution series of DNA extracts were tested in a pre-experiment to avoid the inhibition of PCR, resulting in an optimal dilution of 1:64 for all samples. The efficiencies (Eff) of the amplifications were calculated as described in Töwe et al.56 and resulted in the following values: amoA of ammonia-oxidizing bacteria (AOB) 93–95%, amoA of ammonia-oxidizing archaea (AOA) 91–98%, nirS 99–100%, nirK 94–99% and nosZ 84–85%. The specificity of the amplified products was checked by melting curves of the amplicons and agarose gels.
Table 2.
Protocols for quantitative real-time PCR with thermal profiles, primers and standards used for the different functional genes.
| Target gene | Thermal profile | No. of cycles | Primer | Source of standard |
|---|---|---|---|---|
| amoA AOA | 94 °C–45 s/55 °C–45 s/72 °C–45 s | 40 | amo19F crenamoA16r48x42,61 |
Fosmid clone 54d9 |
| amoA AOB | 94 °C–60 s/58 °C–60 s/72 °C–60 s | 40 | amoA1F amoA2R62 |
Nitrosomonas sp. |
| nirK | 95 °C–15 s/63 °C–30 s/72 °C–30 s | 5a | nirK876 nirK5R63,64 |
Azospirillum irakense |
| 95 °C–15 s/58 °C–30 s/72 °C–30 s | 40 | |||
| nirS | 94 °C–45 s/57 °C–45 s/72 °C–4 s | 40 | cd3aF R3cd65,66 |
Pseudomonas stutzeri |
| nosZ | 95 °C–15 s/65 °C–30 s/72 °C–30 s | 5a | nosZ2F nosZ2R67 |
Pseudomonas fluorescens |
| 95 °C–15 s/60 °C–30 s/72 °C–30 s | 40 |
aTouchdown: −1 °C of the primer annealing temperature per cycle.
Terminal restriction fragment length polymorphism
The diversity of the ammonia oxidizing archaea was assessed by using terminal restriction fragment length polymorphism (t-RFLP) of the amoA gene as described by Töwe et al.57. Same primers and PCR conditions were used for t-RFLP as described above for qPCR, but the forward primer was labeled with 5´-FAM (6-carboxyfluorescein) (Table 2). For the digestion of the PCR product the restriction enzyme MwoI (New England BioLabs, Germany) was used as described by Bannert et al.35. Sequencing was performed on an ABI 3730 DNA Analyzer (Applied Biosystems, USA) and chromatograms were analyzed by using the GeneMapper 3.5 software package (Applied Biosystems, Germany) and T-REX software (http://trex.biohpc.org/) with a binning range of 1 basepare (bp). Data were normalized to percent of the total peak height of a sample. Fragments smaller than 50 bases and terminal restriction fragments (TRFs) contributing <0.5% to the total peak height were excluded57. Samples with peak height smaller than 3% were combined and marked as “others”. TRFs were assigned to the amoA_AOA Feifei-Liu reference database from FunGene58 using TRiFLe software59.
Statistical analysis
Statistical analysis was performed using R 3.4.160. Normal distribution was tested with the Kolmogorov-Smirnoff test; if needed, the data were log-transformed before further analyses. Because of repeated measurements, a linear mixed effect model was calculated (function lme in R-package nlme) and in case of a significant time effect pairwise comparisons were conducted by a Tukey test (function glht in R-package multcomp). To determine treatment-specific differences, we subsequently tested for effects of drought on soil chemical parameters and terminal restriction fragments by comparing treatment with respective controls over the study time by repeated-measures ANOVA. In order to describe treatment effects at single sampling points, we used a paired student’s t-test for independent samples. Changes over time between C and D1 or C and D2 and changes due to the treatment were analyzed with a univariate analysis of variances.
Supplementary information
Acknowledgements
This study was funded by the “Bavarian Climate Programme 2020” of the Bavarian State Ministry of Sciences, Research and Arts within the FORKAST research cooperation “Impact of Climate on Ecosystems and Climatic Adaptation Strategies”. We thank the German Weather Service for the long-term precipitation data. And furthermore, we thank G. Hufnagel for the excellent technical support in measuring soil chemical parameters.
Author Contributions
V.H. collected the soil samples, analyzed the date and wrote the manuscript. E.-M.K. supervised the master student. S.G. made guidance for the writing of the manuscript, revised the manuscript and helped analyzing the data. S.K. performed qPCR analyses. H.S. performed t-RFLP analyses. G.W. supervised the statistical analyses. A.J., C.B. and M.S. reviewed the initial design of the experiment and supervised the experiment.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38147-5.
References
- 1.IPCC. Climate Change 2014: Contribution of Working Group I, II, III to the Fifth Assessment Report of the Intergorvernmental Panel on Climate Change. Synthesis Report 403 (2014).
- 2.Gobiet A, et al. 21st centuryclimate change in the European Alps-A review. Sci. Total Environ. 2014;493:1138–1151. doi: 10.1016/j.scitotenv.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 3.Jentsch A, Beierkuhnlein C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus Geosci. 2008;340:621–628. doi: 10.1016/j.crte.2008.07.002. [DOI] [Google Scholar]
- 4.O´Gorman PAO, Schneider T. The physical basis for increases in precipitation extremes in simulations of 21st-century climate change. PNAS. 2009;106:14773–14777. doi: 10.1073/pnas.0907610106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schloter M, Dilly O, Munch JCC. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003;98:255–262. doi: 10.1016/S0167-8809(03)00085-9. [DOI] [Google Scholar]
- 6.Gordon H, Haygarth PM, Bardgett RD. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 2008;40:302–311. doi: 10.1016/j.soilbio.2007.08.008. [DOI] [Google Scholar]
- 7.Schimel J, Balser TC, Wallenstein M. Microbial Stress-Response Physiology and its Implications for ecosystem function. Ecology. 2007;88:1386–1394. doi: 10.1890/06-0219. [DOI] [PubMed] [Google Scholar]
- 8.Stark JM, Firestone MK. Mechanisms for soil moisture effects on activity of nitrifying bacteria. These include: Mechanisms for Soil Moisture Effects on Activity of Nitrifying Bacteria. Appl. Environ. Microbiol. 1995;61:218–221. doi: 10.1128/aem.61.1.218-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin A, et al. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia. 2004;141:221–35. doi: 10.1007/s00442-004-1519-1. [DOI] [PubMed] [Google Scholar]
- 10.Waldrop M, Firestone M. Seasonal Dynamics of Microbial Community Composition and Function in Oak Canopy and Open Grassland Soils. Microb. Ecol. 2006;52:470–479. doi: 10.1007/s00248-006-9100-6. [DOI] [PubMed] [Google Scholar]
- 11.Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005;20:634–641. doi: 10.1016/j.tree.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Schimel JP, Bennett J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology. 2004;85:591–602. doi: 10.1890/03-8002. [DOI] [Google Scholar]
- 13.Daims H, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528:504. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ollivier J, et al. Nitrogen turnover in soil and global change. FEMS Microbiol. Ecol. 2011;78:3–16. doi: 10.1111/j.1574-6941.2011.01165.x. [DOI] [PubMed] [Google Scholar]
- 15.Harris, R. Effect of Water Potential on Microbial Growth and Activity. In Water Potential Relations in Soil Microbiology (eds. Parr, J. F., Gardner, W. R. & Elliott, L. F.) 23–95, 10.2136/sssaspecpub9.c2 (Soil Science Society of America, 1981).
- 16.Hartmann A, Barnard R, Marhan S, Niklaus P. Effects of drought and N-fertilization on N cycling in two grassland soils. Oecologia. 2013;171:705–17. doi: 10.1007/s00442-012-2578-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser C, et al. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 2010;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasche F, et al. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011;5:389–402. doi: 10.1038/ismej.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan K, et al. Spatial and temporal dynamics of nitrogen fixing, nitrifying and denitrifying microbes in an unfertilized grassland soil. Soil Biol. Biochem. 2017;109:214–226. doi: 10.1016/j.soilbio.2016.11.011. [DOI] [Google Scholar]
- 20.Lipson DA, Schmidt SK, Monson RK. Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology. 1999;80:1623–1631. doi: 10.1890/0012-9658(1999)080[1623:LBMPDA]2.0.CO;2. [DOI] [Google Scholar]
- 21.Bartelheimer M, Poschlod P. The response of grassland species to nitrate versus ammonium coincides with their pH optima. J. Veg. Sci. 2014;25:760–770. doi: 10.1111/jvs.12124. [DOI] [Google Scholar]
- 22.Wolsing M, Priemé A. Observation of high seasonal variation in community structure of denitrifying bacteria in arable soil receiving artificial fertilizer and cattle manure by determining t-RFLP of nir gene fragments. FEMS Mirobiology Ecoloy. 2004;48:261–271. doi: 10.1016/j.femsec.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Jones CM, Graf DRH, Bru D, Philippot L, Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domeignoz-Horta, L. A. et al. The diversity of the N2O reducers matters for the N2O:N2denitrification end-product ratio across an annual and a perennial cropping system. Front. Microbiol. 6 (2015). [DOI] [PMC free article] [PubMed]
- 25.Samad MDS, et al. Phylogenetic and functional potential links pH and N2O emissions in pasture soils. Sci. Rep. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S-Y, Lee S-H, Freeman C, Fenner N, Kang H. Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biol. Biochem. 2008;40:2874–2880. doi: 10.1016/j.soilbio.2008.08.004. [DOI] [Google Scholar]
- 27.Stres B, et al. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol. 2008;66:110–22. doi: 10.1111/j.1574-6941.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicol GW, Leininger S, Schleper C, Prosser JI. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008;10:2966–2978. doi: 10.1111/j.1462-2920.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehtovirta-Morley LE, et al. Identifying potential mechanisms enabling acidophily in the ammonia-oxidising archaeon ‘ Candidatus Nitrosotalea devanaterra’. Appl. Environ. Microbiol. 2016;82:AEM.04031–15. doi: 10.1128/AEM.04031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbold CW, et al. Ammonia-oxidising archaea living at low pH: Insights from comparative genomics. Environ. Microbiol. 2017;19:4939–4952. doi: 10.1111/1462-2920.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prosser JI, Nicol GW. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012;20:523–531. doi: 10.1016/j.tim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Gubry-Rangin C, et al. Niche specialization of terrestrial archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. 2011;108:21206–21211. doi: 10.1073/pnas.1109000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser M, Kleber M, Berhe AA. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015;80:324–340. doi: 10.1016/j.soilbio.2014.10.018. [DOI] [Google Scholar]
- 34.Kuzyakov Y, Xu X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013;198:656–669. doi: 10.1111/nph.12235. [DOI] [PubMed] [Google Scholar]
- 35.Bannert A, et al. Changes in diversity and functional gene abundances of microbial communities involved in nitrogen fixation, nitrification, and denitrification in a tidal wetland versus paddy soils cultivated for different time periods. Appl. Environ. Microbiol. 2011;77:6109–16. doi: 10.1128/AEM.01751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaye JP, Hart SC. Competition for nitrogen between plants and soil microorganisms. Trends Ecol. Evol. 1997;5347:0–4. doi: 10.1016/s0169-5347(97)01001-x. [DOI] [PubMed] [Google Scholar]
- 37.Gubry-Rangin C, et al. Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. Proc. Natl. Acad. Sci. 2015;112:9370–9375. doi: 10.1073/pnas.1419329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Y., Xi, R., Wang, W. & Yao, H. The relative contribution of nitrifiers to autotrophic nitrification across a pH-gradient in a vegetable cropped soil. (2018).
- 39.Kerou M, et al. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. 2016;113:E7937–E7946. doi: 10.1073/pnas.1601212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleeson DB, et al. Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol. Biochem. 2010;42:1888–1891. doi: 10.1016/j.soilbio.2010.06.020. [DOI] [Google Scholar]
- 41.Schleper C. Ammonia oxidation: different niches for bacteria and archaea? ISME J. 2010;4:1092–1094. doi: 10.1038/ismej.2010.111. [DOI] [PubMed] [Google Scholar]
- 42.Leininger S, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 43.Fierer N, Schimel JP, Holden PA. Variations in microbial community composition through two soil depth profiles. Microbiology. 2003;35:167–176. [Google Scholar]
- 44.Cui M, Caldwell M. A large emphemeral release of nitrogen upon wetting of dry soil and corresponding root responses in the field. Plant Soil. 1997;191:291–299. doi: 10.1023/A:1004290705961. [DOI] [Google Scholar]
- 45.Scheu S, Parkinson D. Changes in bacterial and fungal biomass C, bacterial and fungal biovolume and ergosterol content after drying, remoistening and incubation of different layers of cool termperate forest soils. Soil Biol. Biochem. 1994;26:1515–1525. doi: 10.1016/0038-0717(94)90093-0. [DOI] [Google Scholar]
- 46.Sorensen LH. Rate of decomposition of oragnic matter in soil as influenced by repeated air drying-rewetting and repeated additions of organic material. Soil Biol. Biochem. 1974;6:287–292. doi: 10.1016/0038-0717(74)90032-7. [DOI] [Google Scholar]
- 47.Birch HF. The effect of soil drying on humus decompsition and nitrogen availability. Plant Soil. 1958;10:9–31. doi: 10.1007/BF01343734. [DOI] [Google Scholar]
- 48.Horz H-P, Barbrook A, Field CB, Bohannan BJM. Ammonia-oxidizing bacteria respond to multifactorial global change. Proc. Natl. Acad. Sci. USA. 2004;101:15136–41. doi: 10.1073/pnas.0406616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alves, R. J. E. et al. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 1–12, 10.1038/ismej.2013.35 (2013). [DOI] [PMC free article] [PubMed]
- 50.Wang C, et al. Climate change amplifies gross nitrogen turnover in montane grasslands of Central Europe in both summer and winter seasons. Glob. Chang. Biol. 2016;22:2963–2978. doi: 10.1111/gcb.13353. [DOI] [PubMed] [Google Scholar]
- 51.Grant K, Kreyling J, Beierkuhnlein C, Jentsch A. Importance of Seasonality for the Response of a Mesic Temperate Grassland to Increased Precipitation Variability and Warming. Ecosystems. 2017;20:1–14. doi: 10.1007/s10021-017-0122-3. [DOI] [Google Scholar]
- 52.Jentsch A, Beierkuhnlein C. Simulating the Future - Responses of Ecosystems, Key Species, and European Provenances to Expected Climatic Trends and Events. Nov. Acta Leopoldina NF. 2010;384:89–98. [Google Scholar]
- 53.Walter J, et al. Increased rainfall variability reduces biomass and forage quality of temperate grassland largely independent of mowing frequency. Agric. Ecosyst. Environ. 2012;148:1–10. doi: 10.1016/j.agee.2011.11.015. [DOI] [Google Scholar]
- 54.Glaser B, Jentsch A, Kreyling J, Beierkuhnlein C. Soil-moisture change caused by experimental extreme summer drought is similar to natural inter-annual variation in a loamy sand in CentralEurope. Plant Nutr. an Soil Sci. 2013;176:27–34. doi: 10.1002/jpln.201200188. [DOI] [Google Scholar]
- 55.Grant K, Kreyling J, Dienstbach LFH, Beierkuhnlein C, Jentsch A. Water stress due to increased intra-annual precipitation variability reduced forage yield but raised forage quality of a temperate grassland. Agric. Ecosyst. Environ. 2014;186:11–22. doi: 10.1016/j.agee.2014.01.013. [DOI] [Google Scholar]
- 56.Töwe S, et al. Abundance of microbes involved in nitrogen transformation in the rhizosphere of Leucanthemopsis alpina (L.) Heywood grown in soils from different sites of the Damma glacier forefield. Microb. Ecol. 2010;60:762–70. doi: 10.1007/s00248-010-9695-5. [DOI] [PubMed] [Google Scholar]
- 57.Töwe S, et al. Improved protocol for the simultaneous extraction and column-based separation of DNA and RNA from different soils. Microbiol. Methods. 2011;84:406–412. doi: 10.1016/j.mimet.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 58.Fish J, et al. FunGene: the functional gene pipeline and repository. Frontiers in Microbiology. 2013;4:291. doi: 10.3389/fmicb.2013.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Junier P, Junier T, Witzel KP. TRiFLe, a program for in silico terminal restriction fragment length polymorphism analysis with user-defined sequence sets. Appl. Environ. Microbiol. 2008;74:6452–6456. doi: 10.1128/AEM.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Core Team. R: A Language and Environment for Statistical Computing. (2012).
- 61.Schauss K, et al. Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009;11:446–56. doi: 10.1111/j.1462-2920.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 62.Rotthauwe J, Witzel K, Liesack W. The Ammonia Monooxygenase Structural Gene amoA as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997;63:4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braker G. Fesefeldt, a & Witzel, K. P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998;64:3769–75. doi: 10.1128/aem.64.10.3769-3775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henry S, et al. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods. 2004;59:327–335. doi: 10.1016/j.mimet.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Michotey V, Méjean V, Bonin P. Comparison of Methods for Quantification of Cytochrome cd 1 -Denitrifying Bacteria in Environmental Marine Samples. Appl. Environ. Microbiol. 2000;66:1564–1571. doi: 10.1128/AEM.66.4.1564-1571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Throbäck IN, Enwall K, Jarvis A, Hallin S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004;49:401–17. doi: 10.1016/j.femsec.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 67.Henry S, Bru D, Stres B, Hallet S, Philippot L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006;72:5181–9. doi: 10.1128/AEM.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.