Abstract
Intermittent preventive treatment with sulphadoxine-pyrimethamine (SP) and SP plus azithromycin (SPAZ) reduces low birthweight (<2,500 g) in women without malarial and reproductive tract infections. This study investigates the impact of SPAZ on associations between plasma biomarkers of inflammation and angiogenesis and adverse pregnancy outcomes in 2,012 Papua New Guinean women. Concentrations of C-reactive protein (CRP), α-1-acid glycoprotein (AGP), soluble endoglin (sEng), soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF) were measured at enrolment and delivery in a trial comparing SPAZ to SP plus chloroquine (SPCQ). At antenatal enrolment higher CRP (adjusted odds ratio 1.52; 95% confidence interval [CI] 1.03–2.25), sEng (4.35; 1.77, 10.7) and sFlt1 (2.21; 1.09, 4.48) were associated with preterm birth, and higher sEng with low birthweight (1.39; 1.11,3.37), in SPCQ recipients only. Increased enrolment sFlt1:PlGF ratios associated with LBW in all women (1.46; 1.11, 1.90). At delivery, higher AGP levels were strongly associated with low birthweight, preterm birth and small-for-gestational age babies in the SPCQ arm only. Restricting analyses to women without malaria infection did not materially alter these relationships. Women receiving SPAZ had lower delivery AGP and CRP levels (p < 0.001). SPAZ may protect against adverse pregnancy outcomes by reducing inflammation and preventing its deleterious consequences, including dysregulation of placental angiogenesis, in women with and without malarial infection.
Introduction
Adverse pregnancy outcomes including low birth weight (LBW, <2500 g), preterm birth (PTB, <37 gestational weeks) and small-for-gestational-age (SGA) are frequent in low- and middle-income countries (LMIC)1. Malaria, sexually transmitted infections, and urinary tract infections are common and contribute to adverse pregnancy outcomes in these settings1–3. The burden of LBW is highest in sub-Saharan Africa and South Asia4, regions where facilities to care for LBW babies are scarce and neonatal death commonly ensues5.
In pregnancy, inflammation, as measured through maternal plasma C-reactive protein (CRP) levels, has been associated with SGA6, pre-eclampsia7, and PTB8, with most evidence originating from high-income settings. In LMICs, important drivers of increased CRP include placental malaria, Chlamydia trachomatis infection, and chronic inflammation at distal sites (e.g. periodontitis)9–11. Spontaneous PTB is described as a multifactorial syndrome, and inflammation, whether sterile or as a result of infection, is thought to be an important contributing factor12.
Inflammation and angiogenesis are closely linked13. Malaria infection and concomitant inflammation have been associated with increased maternal serum levels of the antiangiogenic protein endoglin (sEng; expressed by vascular endothelium and syncytiotrophoblast) and reduced levels of proangiogenic placental growth factor (PlGF), resulting in placental vascular remodeling and fetal growth restriction14.
Intermittent preventive treatment in pregnancy (IPTp), the presumptive administration of antimalarials at antenatal clinic visits, decreases placental malaria and improves birthweights15. IPTp was introduced as many pregnant women with malaria are asymptomatic and point-of-care tests missed placental infections16. Currently recommended is monthly sulphadoxine-pyrimethamine (SP) from second trimester, but new IPTp candidate regimens are needed17. Two clinical trials of SP plus azithromycin (AZ) demonstrated reduction in the risk of PTB and LBW18,19. In Papua New Guinea (PNG), women randomised to three courses of SP and AZ (1 g twice daily for 2 days) had a lower risk of LBW (26% relative risk reduction, P = 0.005) and PTB (38%, P = 0.01) compared to control (single dose of SP plus chloroquine for 3 days at first antenatal visit, as per national policy)18. In Malawi, monthly courses of SP plus AZ (1 g) given at first and second treatment visits in addition to routine SP reduced the risk of LBW (relative risk reduction 39%, P = 0.04) and PTB (34%, P = 0.01) compared to women receiving two monthly SP treatments only19.
AZ is a macrolide with activity against Plasmodium spp. parasites as well syphilis, C. trachomatis and Neisseria gonorrhoeae, and urinary tract infections20,21. Structurally related to tacrolimus, it has immunomodulatory and anti-inflammatory properties, most studied in chronic lung diseases22. SP and SPAZ reduce LBW and PTB in the context of falling malaria prevalence, in areas with high-level resistance to SP, and in absence of malaria infection23–25. This suggests antibacterial and anti-inflammatory properties of SP and AZ may play a significant role.
Inflammation due to infectious and non-infectious processes may lead to adverse pregnancy outcomes and may do so it part by altering placental angiovasculogenesis. Surrogate measures of inflammation (CRP, α-1-Acid glycoprotein [AGP]) and angiogenesis sEng, soluble fms-like tyrosine kinase [sFlt-1], and PlGF are biomarkers that have been associated with pregnancy outcomes. We hypothesised that SPAZ prevents adverse pregnancy outcomes by reducing and preventing inflammation and dysregulation of placental angio- and vasculogenesis. We examined the relationship between biomarkers of inflammation and angiogenesis at antenatal enrolment or at delivery and adverse pregnancy outcomes. We then determined whether these relationships differed by treatment arm and persisted amongst women without malarial infection. This secondary analysis was performed drawing on data and samples of the aforementioned PNG-IPTp trial which was conducted at several health centres situated in Madang Province on the North Coast of PNG from 2009 to 201318. The majority of pregnant women enrolled in the trial resided in rural and peri-urban areas where there is perennial transmission of P. falciparum and P. vivax malaria. The region is characterised by a high burden of LBW (17%), and a high prevalence of bacterial sexually transmitted infections and undernutrition in pregnancy26–29.
Results
Study population
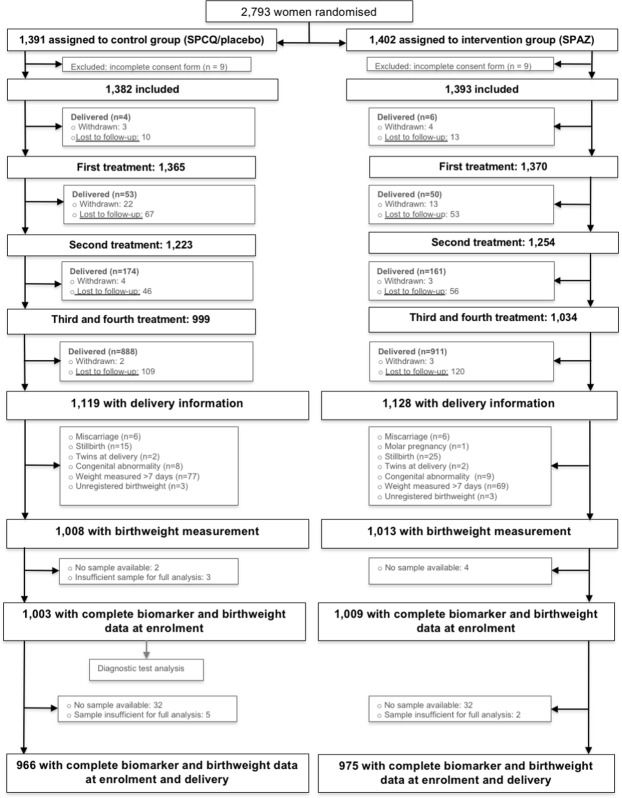
Of 2,973 participants in the trial, 2,021 completed birthweight follow-up and delivered a live singleton congenitally normal infant18. Biomarker levels at enrolment were measured for 2,012 of these women (99.6%), and 1,941 (96.5%) had biomarker assessments at both enrolment and delivery (Fig. 1).
Figure 1.
Participant flow chart. SPCQ, sulphadoxine-pyrimethamine plus chloroquine; SPAZ, sulphadoxine-pyrimethamine plus azithromycin.
Women in each group had similar characteristics at enrolment (Table 1), apart from higher CRP levels amongst women randomised to SPAZ (p = 0.025).
Table 1.
Cohort characteristics and birth outcomes, by treatment arm.
| Variable | N | SPCQ | SPAZ | P |
|---|---|---|---|---|
| (n = 1,003) | (n = 1,009) | |||
| Enrolment | ||||
| Smoker | 2,012 | 18.3 | 20.4 | 0.22 |
| Malaria infectiona | 2,012 | 13.8 | 13.5 | 0.86 |
| Primigravida | 2,009 | 51.7 | 49.2 | 0.27 |
| Haemoglobin (g/L) | 1,927 | 97 (96, 98) | 97 (96, 98) | 0.41 |
| Gestational age by ultrasound (weeks) | 1,314 | 21.7 (21.4, 22.0) | 21.8 (21.4, 22.1) | 0.91 |
| Mid-upper arm circumference (cm) | 1,970 | 23.9 (23.8, 24.1) | 24.0 (23.8, 24.2) | 0.44 |
| Maternal age (years) | 2,012 | 24.7 (24.3, 25.0] | 24.4 (24.0, 24.8] | 0.38 |
| CRP (mg/L) | 2,012 | 1.3 (1.2, 1.4) | 1.5 (1.4, 1.7) | 0.025 |
| AGP (mg/L) | 222 (211, 232) | 220 (209, 232) | 0.85 | |
| sEng (pg/ml) | 14.8 (14.1, 15.5) | 14.6 (14.0, 15.4) | 0.74 | |
| sFlt-1(ng/ml) | 2.6 (2.5, 2.8) | 2.5 (2.4, 2.7) | 0.39 | |
| PIGF (pg/ml) | 162 (155, 171) | 156 (149, 164) | 0.27 | |
| Delivery | (n = 966) | (n = 975) | ||
| Low birthweight | 2,012 | 17.5 | 12.7 | 0.003 |
| Preterm birth (<37 weeks) | 1,314 | 10.6 | 6.8 | 0.013 |
| Small-for-gestational-age | 1,314 | 25.1 | 23.9 | 0.61 |
| Female baby | 2,006 | 56.8 | 54.7 | 0.36 |
| Birthweight (g) | 2,012 | 2922 (510) | 2964 (445) | 0.05 |
| Gestational age by ultrasound (weeks) | 1,314 | 39.0 | 39.3 | 0.013 |
| CRP (mg/L) | 1,941 | 1.3 (1.2, 1.4) | 1.0 (0.9, 1.1) | 0.003 |
| AGP (mg/L) | 175 (167, 183) | 162 (154, 169) | 0.015 | |
| sEng (pg/ml) | 23.0 (21.7, 24.3) | 22.6 (21.4, 24.0) | 0.75 | |
| sFlt-1 (ng/ml) | 4.9 (4.5, 5.3) | 4.8 (4.4, 5.2) | 0.74 | |
| PlGF (pg/ml) | 74.8 (71.6, 78.1) | 76.3 (73.1, 79.6) | 0.50 | |
Continuous data are presented arithmetic mean (95% confidence interval) [clinical variables] or as geometric mean (95% confidence interval) [biomarker levels]. Categorical data are presented as %. AGP, α-1-acid glycoprotein; CRP, C-reactive protein; PlGF, placental growth factor; sEng; soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase; SPCQ, sulphadoxine-pyrimethamine plus chloroquine; SPAZ, sulphadoxine-pyrimethamine plus azithromycin.
aPeripheral parasitaemia by light microscopy and/or real-time polymerase chain reaction (Plasmodium falciparum, P. vivax).
Pregnancy outcomes
The burden of adverse pregnancy outcomes was high. There were 303 women who delivered LBW infants (15.1%) (Table 1). Amongst 1,314 ultrasound-dated pregnancies, 8.7% (n = 114) had spontaneous PTB, and 24.5% (n = 322) were SGA. SPAZ reduced the risk of LBW and PTB. Women randomised to SPAZ had lower levels of CRP (P = 0.003) and AGP (P = 0.015) at delivery (Table 1).
Relationship between biomarker levels and clinical factors
At enrolment, women with clinical signs of infection (feeling unwell, history of self-reported fever in preceding 24 hours) and anaemia had higher CRP levels, and peripheral malaria infection was associated with increased CRP (P < 0.001), lower sFlt-1 (P = 0.017), and reduced levels of PlGF (P = 0.006) (Supplemental Tables 1, 2). Women with a mid-upper arm circumference < 23 cm (a clinical marker of undernutrition) had lower levels of CRP (P < 0.007) and higher levels of sFlt-1 (P = 0.018). A mean arterial pressure ≥ 90 mmHg at antenatal enrolment was associated with increased sEng (P < 0.001) (Supplemental Tables 1, 2).
At delivery, all malariometric indices were associated with increased levels of CRP, and active placental malaria with increased AGP (P = 0.012) (Supplemental Table 1). Evidence of past infection on placental histology was associated with lower levels of sEng (P = 0.003), sFlt-1 (P = 0.013) and reduced sFlt-1:PlGF ratios (P = 0.008) (Supplemental Table 2).
Biomarker levels at enrolment and adverse pregnancy outcomes
Assessing all women, higher levels of sEng were associated with LBW and PTB, sFlt-1 with LBW, higher sFlt-1:PlGF ratios with LBW, and lower PlGF levels with SGA (Table 2). Stratification by trial arm revealed differences in associations between biomarker levels and adverse pregnancy outcomes. Significant associations were largely restricted to women who had been randomised to SPCQ; amongst them, higher enrolment CRP levels were associated with PTB, increases in sEng were associated with LBW and PTB, and higher levels of sFlt-1 with PTB (Table 2). Increasing sFlt-1:PlGF ratios were associated with LBW overall and by treatment arm.
Table 2.
Adjusted odds ratios (aOR) for biomarker levels at enrolment (log-transformed) and adverse birth outcomes, overall and by trial arm.
| Outcome | SPCQ | P | SPAZ | P | All | P |
|---|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | ||||
| Low birthweight a | 175/1,003 | 128/1009 | 303/2,012 | |||
| CRP (mg/L) | 1.14 (0.87, 1.48) | 0.32 | 0.98 (0.72, 1.32) | 0.87 | 1.05 (0.86, 1.27) | 0.64 |
| AGP (mg/L) | 0.94 (0.58, 1.51) | 0.81 | 1.70 (0.96, 3.01) | 0.07 | 1.21 (0.84, 1.74) | 0.30 |
| sEng (pg/ml) | 1.93 (1.11, 3.37) | 0.022 | 1.23 (0.69, 2.20) | 0.48 | 1.58 (1.07, 2.35) | 0.024 |
| sFlt-1 (ng/ml) | 1.39 (0.89, 2.16) | 0.15 | 1.70 (0.96, 2.99) | 0.07 | 1.47 (1.04, 2.09) | 0.028 |
| PlGF (pg/ml) | 0.70 (0.42, 1.16) | 0.15 | 0.75 (0.42, 1.33) | 0.32 | 0.75 (0.51, 1.09) | 0.12 |
| sFlt-1/PlGF | 1.48 (1.04, 2.20) | 0.030 | 1.54 (1.02, 2.33) | 0.040 | 1.46 (1.11, 1.90) | 0.006 |
| Preterm birth b | 69/649 | 45/665 | 114/1,314 | |||
| CRP (mg/L) | 1.52 (1.03, 2.25) | 0.035 | 0.86 (0.53, 1.39) | 0.54 | 1.20 (0.86, 1.62) | 0.24 |
| AGP (mg/L) | 0.92 (0.46, 1.86) | 0.80 | 0.99 (0.43, 2.24) | 0.97 | 0.96 (0.57, 1.62) | 0.86 |
| sEng (pg/ml) | 4.35 (1.77, 10.7) | 0.001 | 1.37 (0.52, 3.62) | 0.53 | 2.49 (1.31, 4.74) | 0.006 |
| sFlt-1 (ng/ml) | 2.21 (1.09, 4.48) | 0.028 | 1.00 (0.41, 2.41) | 0.99 | 1.64 (0.96, 2.82) | 0.07 |
| PlGF (pg/ml) | 1.37 (0.59, 3.17) | 0.47 | 0.66 (0.23, 1.87) | 0.43 | 1.02 (0.53, 1.95) | 0.95 |
| sFlt-1/PlGF | 1.44 (0.83, 2.51) | 0.20 | 1.19 (0.61, 2.33) | 0.61 | 1.34 (0.88, 2.05) | 0.17 |
| SGA b | 163/649 | 159/665 | 322/1,314 | |||
| CRP (mg/L) | 1.05 (0.79, 1.39) | 0.74 | 0.94 (0.70, 1.25) | 0.68 | 0.98 (0.80, 1.19) | 0.84 |
| AGP (mg/L) | 1.04 (0.64, 1.70) | 0.87 | 1.36 (0.82, 2.24) | 0.24 | 1.18 (0.84, 1.67) | 0.35 |
| sEng (pg/ml) | 1.07 (0.61, 1.89) | 0.85 | 1.03 (0.58, 1.81) | 0.94 | 1.07 (0.78, 1.59) | 0.78 |
| sFlt-1 (ng/ml) | 0.97 (0.59, 1.60) | 0.90 | 1.12 (0.66, 1.88) | 0.62 | 1.04 (0.72, 1.49) | 0.83 |
| PlGF (pg/ml) | 0.54 (0.29, 1.01) | 0.05 | 0.61 (0.33, 1.13) | 0.12 | 0.59 (0.38, 0.91) | 0.016 |
| sFlt-1/PlGF | 1.27 (0.85, 1.90) | 0.25 | 1.34 (0.90, 2.00) | 0.16 | 1.29 (0.97, 1.71) | 0.08 |
AGP, α-1-acid glycoprotein; CI, confidence interval; CRP, C-reactive protein; PlGF, placental growth factor; sEng; soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase; SPCQ, sulphadoxine-pyrimethamine plus chloroquine; SPAZ, sulphadoxine-pyrimethamine plus azithromycin. NS, not significant, defined as p ≥ 0.05.
aOdds ratios and 95% confidence intervals were estimated using logistic regression and adjusted for sex, bed net use, mid-upper arm circumference, recruitment clinic, height, partner’s work status, number of antenatal visits, timing of birthweight measurement, and fundal height at biomarker measurement.
bOdds ratios and 95% confidence intervals were estimated using logistic regression and adjusted for sex, bed net use, mid-upper arm circumference, recruitment clinic, height, partner’s work status, number of antenatal visits, and gestational age at biomarker measurement by ultrasound.
When we examined associations between abnormal biomarker levels at enrolment and birthweight (continuous variable) (Supplemental Table 3), women with elevated CRP levels (≥5.0 mg/L) who were randomised to SPCQ had an adjusted mean reduction in birthweight of 96 g (95% CI 13, 179) (P = 0.023). No other significant associations were observed.
Relationship of biomarker levels at birth with treatment arm and adverse pregnancy outcomes
At birth biomarker data were available for 966 women (627 with PTB data) in the SPCQ arm, and 975 (646 with PTB data) in the SPAZ arm.
Women randomised to SPAZ had lower CRP and AGP levels at delivery (Table 1).
Higher levels of AGP at delivery were strongly associated with LBW, PTB and SGA. Upon stratification by trial arm, these associations were confined to women randomised to SPCQ (Table 3). Most pronounced effects on mean birthweight were observed amongst women with an elevated AGP at birth: birthweight reductions in women with raised AGP levels were similar in women with normal and concomitantly raised CRP levels (Fig. 2). When stratifying by treatment arm raised AGP levels were associated with pronounced reductions in birthweight (adjusted mean difference 180 g, 95% CI 111, 251) amongst SPCQ recipients (Fig. 2). A raised CRP was associated with reduced risk of PTB amongst SPAZ recipients (0.52; 0.28, 0.95; P = 0.035).
Table 3.
Adjusted odds ratios (aOR) for biomarker levels at delivery (log-transformed) and adverse birth outcomes, overall and by trial arm.
| Outcome | SPCQ | P | SPAZ | P | All | P |
|---|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | ||||
| Low birthweight a | 168/966 | 124/975 | 292/1,941 | |||
| CRP (mg/L) | 1.17 (0.91, 1.51) | 0.23 | 0.97 (0.73, 1.30) | 0.85 | 1.10 (0.91, 1.33) | 0.32 |
| AGP (mg/L) | 2.40 (1.38, 4.18) | 0.002 | 1.21 (0.67, 2.22) | 0.53 | 1.79 (1.20, 2.68) | 0.004 |
| sEng (pg/ml) | 1.79 (1.12, 2.88) | 0.016 | 1.61 (0.95, 2.72) | 0.08 | 1.69 (1.19, 2.40) | 0.003 |
| sFlt-1 (ng/ml) | 1.05 (0.76, 1.44) | 0.73 | 1.42 (0.95, 2.11) | 0.08 | 1.18 (0.92, 1.51) | 0.18 |
| PlGF (pg/ml) | 0.65 (0.36, 1.17) | 0.14 | 0.51 (0.25, 1.03) | 0.06 | 0.58 (0.37, 0.91) | 0.018 |
| sFlt-1/PlGF | 1.18 (0.87, 1.60) | 0.27 | 1.62 (1.12, 2.34) | 0.010 | 1.34 (1.06, 1.69) | 0.014 |
| Preterm birth b | 68/627 | 44/646 | 112/1,273 | |||
| CRP (mg/L) | 1.27 (0.85, 1.91) | 0.22 | 0.69 (0.42, 1.13) | 0.14 | 1.02 (0.76, 1.39) | 0.87 |
| AGP (mg/L) | 5.27 (2.17, 12.08) | <0.001 | 0.73 (0.29, 1.82) | 0.48 | 2.10 (1.14, 3.88) | 0.017 |
| sEng (pg/ml) | 2.12 (1.05, 4.26) | 0.036 | 2.59 (1.04, 6.46) | 0.041 | 2.22 (1.28, 3.82) | 0.004 |
| sFlt-1 (ng/ml) | 1.11 (0.68, 1.82) | 0.67 | 2.10 (1.08, 4.10) | 0.029 | 1.41 (0.96, 2.07) | 0.08 |
| PlGF (pg/ml) | 0.34 (0.12, 0.95) | 0.0393 | 0.46 (0.14, 1.50) | 0.20 | 0.37 (0.17, 0.80) | 0.011 |
| sFlt-1/PlGF | 1.42 (0.89, 2.28) | 0.14 | 2.27 (1.23,4.17) | 0.008 | 1.71 (1.19, 2.46) | 0.004 |
| SGA b | 160/627 | 155/646 | 315/1,273 | |||
| CRP (mg/L) | 1.15 (0.88, 1.52) | 0.30 | 1.01 (0.76, 1.35) | 0.87 | 1.07 (0.88, 1.31) | 0.46 |
| AGP (mg/L) | 2.93 (1.60, 5.36) | <0.001 | 1.23 (0.70, 2.14) | 0.47 | 1.84 (1.23, 2.75) | 0.003 |
| sEng (pg/ml) | 1.57 (0.98, 2.50) | 0.05 | 1.11 (0.70, 1.76) | 0.72 | 1.32 (0.95, 1.83) | 0.10 |
| sFlt-1 (ng/ml) | 1.12 (0.79, 1.58) | 0.51 | 1.16 (0.81, 1.67) | 0.45 | 1.13 (0.88, 1.44) | 0.33 |
| PlGF (pg/ml) | 0.90 (0.48, 1.67) | 0.73 | 0.91 (0.48, 1.73) | 0.77 | 0.91 (0.59, 1.42) | 0.68 |
| sFlt-1/PlGF | 1.14 (0.82, 1.57) | 0.42 | 1.16 (0.83, 1.62) | 0.40 | 1.14 (0.91, 1.43) | 0.27 |
AGP, α-1-Acid glycoprotein; CI, confidence interval; CRP, C-reactive protein; PlGF, placental growth factor; sEng; soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase; SPCQ, sulphadoxine-pyrimethamine plus chloroquine; SPAZ, sulphadoxine-pyrimethamine plus azithromycin. NS, not significant, defined as p ≥ 0.05.
aOdds ratios and 95% confidence intervals were estimated using logistic regression and adjusted for sex, bed net use, mid-upper arm circumference, recruitment clinic, height, partner’s work status, number of antenatal visits, and timing of birthweight measurement.
bOdds ratios and 95% confidence intervals were estimated using logistic regression and adjusted for sex, bed net use, mid-upper arm circumference, recruitment clinic, height, partner’s work status, and number of antenatal visits.
Figure 2.
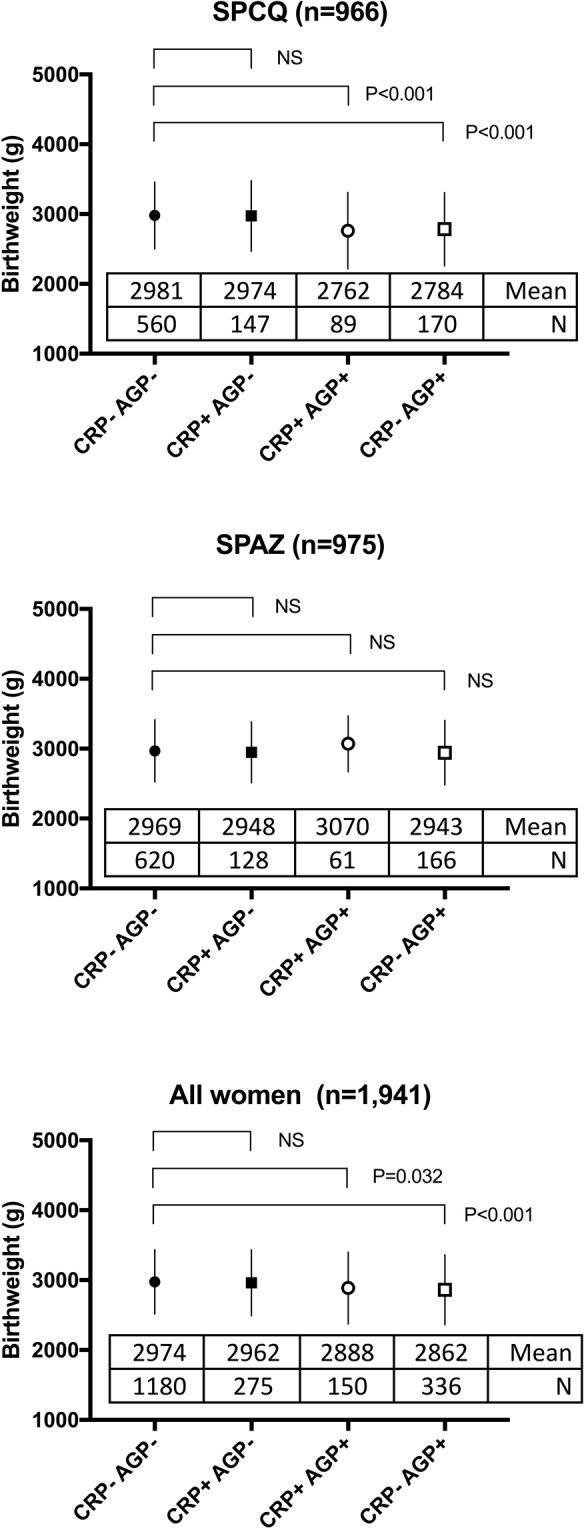
Association between inflammation category at delivery and birthweight, by treatment arm and overall. Figures indicate mean (circles or squares) and standard deviation (whiskers). Numbers show mean birthweight (g) and numbers of women in each group, based on presence or absence of elevation in C-reactive protein (CRP) or α-1-acid glycoprotein (AGP). SPAZ; sulphadoxine-pyrimethamine plus azithromycin; SPCQ, sulphadoxine-pyrimethamine plus chloroquine.
Angiogenic markers exhibited differential associations with adverse birth outcomes when assessed by trial arm. Higher levels of sEng associated with LBW and PTB overall, with LBW amongst SPCQ recipients, and with PTB in both SPCQ and SPAZ recipients. Higher sFlt-1:PlGF ratios were associated with LBW and PTB amongst SPAZ recipients only (Table 3).
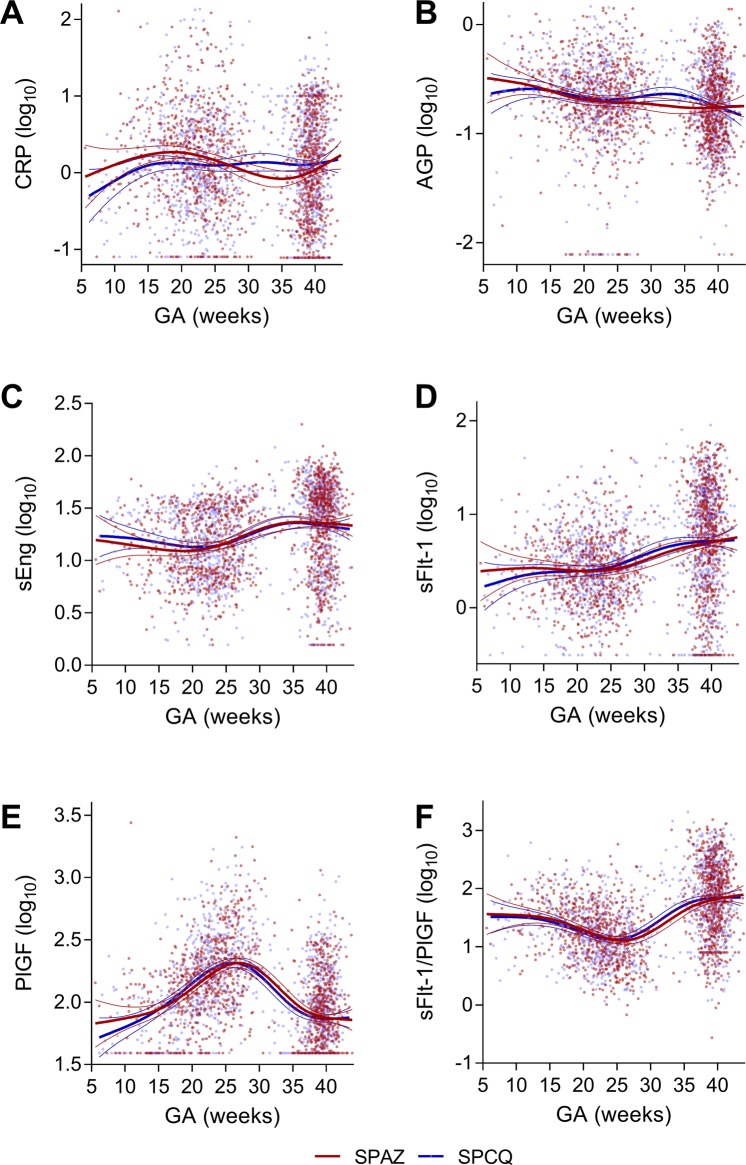
Multilevel linear mixed effects models did not show any significant differences in biomarker levels with gestational age between treatment arms. Statistically significant differences between treatment arms for AGP (and CRP, data not shown) were noted in the 31–35 gestational weeks range (Supplemental Fig. 1). Biomarker levels were further described pictorially using generalised additive models (Fig. 3).
Figure 3.
Longitudinal changes of biomarker levels according to treatment arm. Biomarkers levels (log10) measured at enrolment and delivery (1,273 pairs) were plotted by gestational age at measurement. (A) c-reactive protein (CRP); (B) α-1-acid glycoprotein (AGP); (C) soluble endoglin (sEng); (D) soluble fms-like tyrosine kinase (sFlt-1); (E), placental growth factor (PlGF); and (F) sFlt-1/PlGF. Curves indicate the best fit and 95% confidence bands of the general additive models. Data points and regression lines are coloured by treatment arm (red, sulphadoxine-pyrimethamine plus azithromycin, SPAZ; blue, sulphadoxine-pyrimethamine plus chloroquine, SPCQ).
Associations between biomarker levels and adverse pregnancy outcomes in women without malarial infection
Malaria infection may alter biomarker levels, and differences in antimalarial treatment efficacy may explain trends observed in Tables 2 and 3. In order to investigate the hypothesis that SPAZ reduces LBW through mechanisms other than malaria prevention and treatment we reassessed associations between biomarker levels at enrolment or delivery and adverse pregnancy outcomes amongst women who tested negative for malaria infection. Malaria infection was defined as P. falciparum and/or P. vivax detected at least once during pregnancy. Enrolment biomarker data from 1,540 women and delivery biomarkers from 1,481 women were analysed (Supplemental Tables 4,5).
Most associations observed in the overall cohort persisted in this subset of women unlikely to have had malaria infection (Supplemental Table 4). At enrolment and amongst SPCQ recipients, higher levels of sEng (adjusted odds ratio [aOR] 3.77; 95% CI 1.32, 10.7; P = 0.030; n = 484), CRP (2.30; 1.39, 3.79; P = 0.001), and sFlt-1 (2.50; 1.10, 5.60; P = 0.029) were associated with PTB. Higher enrolment sFlt-1:PlGF ratio was associated with LBW overall (1.54; 1.13, 2.10; P = 0.006), and amongst SPCQ recipients (1.56; 1.02, 2.37; P = 0.039). Higher levels of PlGF were associated with reduced odds of SGA in all women (0.56; 0.34, 0.94; P = 0.026).
Similarly, at delivery higher levels of AGP were associated with LBW (aOR 2.47, 95% CI 1.29, 4.73), P = 0.007), PTB (4.27; 1.53, 11.7; P = 0.005) and SGA (3.94; 1.91, 8.14; P <0.001) amongst SPCQ recipients without malaria infection (Supplemental Table 5). Higher levels of sEng were associated with LBW (2.15; 1.40, 3.29; P = 0.001) and PTB (3.06; 1.55, 6.07; P = 0.001) in all women, and with SGA amongst SPCQ recipients (1.82; 1.04, 3.18; P = 0.035). Higher levels of PlGF associated with reduced odds of LBW in all women (0.53; 0.31, 0.91; P = 0.021), and with reduced odds of PTB amongst SPCQ recipients (0.29; 0.09, 0.97; P = 0.045), and higher sFlt-1:PlGF ratios were associated with LBW amongst SPAZ recipients (1.70; 1.10, 2.63; P = 0.017).
Prediction of adverse pregnancy outcomes
The role of biomarker measurements at first antenatal visit as tests to predict LBW, PTB or SGA was assessed amongst 1,003 women randomised to SPCQ (Supplemental Table 6). AUC readings for individual biomarkers at enrolment in relation to LBW, PTB or SGA were all below < 0.65, indicating poor diagnostic performance. The highest AUC, for the prediction of PTB, was observed for sEng (0.631). Combinations of biomarkers did not markedly improve the AUC, and neither did the addition of measured clinical factors, such as mid-upper arm circumference (all AUC <0.7).
Discussion
Biomarkers of inflammation, infection and angiogenesis at first antenatal visit and delivery were associated with adverse pregnancy outcomes in women enrolled in a randomised controlled trial of malaria prevention in PNG. Most associations of biomarker levels at enrolment with adverse pregnancy outcomes were observed amongst women who were randomised to SPCQ, the control treatment. For instance, elevated levels of CRP, sEng, and sFlt-1 at antenatal enrolment were associated with PTB only amongst SPCQ recipients. At delivery, SPAZ was associated with reduced maternal serum levels of CRP and AGP. Raised delivery levels of AGP were strongly associated with LBW, PTB and a marked reduction in mean birthweight in SPCQ recipients only.
SPAZ demonstrated superior antimalarial efficacy compared to SPCQ18, and differences in associations between biomarkers and adverse pregnancy outcomes may be attributable to differential antimalarial efficacy alone. However, most treatment-specific associations between biomarkers and birth outcomes persisted in analyses that excluded women with detectable malaria infection. This suggests that SPAZ ablates other, malaria-independent, pathological inflammatory and angiogenic processes. These may include clearance of other causes of inflammation, such as sexually transmitted, reproductive tract and urinary tract infections20; preventing or treating ascending infection or infections at distal sites, such as periodontal disease30; or impacts on the vaginal or gut microbiome31. Additionally, azithromycin has anti-inflammatory actions that may decrease placental inflammation of infectious origin or indeed treat sterile inflammatory conditions32, thereby improving fetal growth and prolonging pregnancy duration.
Women in the control arm received once-off SPCQ treatments, whereas women in the SPAZ arm also received second and third treatments. Higher numbers of SP doses are associated with improved birth outcomes after adjusting for antimalarial efficacy, and SP is known to have antibacterial properties that may be of benefit23. The trial design precluded assessment of the possible independent impacts of AZ and SP on biomarker levels and adverse pregnancy outcomes. A trial of SPAZ in Malawi, which compared it to SP-IPTp rather than SPCQ, confirmed benefit of adding AZ to SP-IPTp for prevention of LBW and PTB19. A new trial which compares IPTp with dihydroartemisinin-piperaquine with or without AZ against SP-IPTp will permit further assessment of AZ-specific benefits for pregnancy outcomes and impacts on biomarkers (Clinicaltrials.gov NCT03208179).
A meta-analysis of the role of presumptive antibiotic treatment in second and third trimester for the prevention of PTB suggested no benefit33. However, trials such as the PNG trial were excluded as women in the control arm received active treatment19. The only LMIC trial of AZ for the prevention of PTB included in the meta-analysis showed no benefit34, but arguably the study should also have been excluded as women in the control arm received SP-IPTp as part of routine antenatal care. It is likely that the contribution of infection to PTB is high in low-income settings, and that the positive effect of SPAZ for PTB prevention in our trial is mediated through a broader reduction in the infective or inflammatory burden in participants. A reappraisal of evidence from high PTB burden settings on the relationship between use of agents with antibacterial and anti-inflammatory activity (including IPTp) and pregnancy outcomes appears warranted.
Presence of inflammation at either enrolment or delivery, indicated by levels of CRP and AGP, was associated with adverse outcomes amongst women who did not receive SPAZ. At enrolment, CRP was more strongly associated with PTB, whilst at delivery raised levels of AGP most strongly associated with LBW and PTB. Chronic inflammatory processes, which AGP is thought to reflect more prominently given its slower rise and longer half-life35, may be the main driver for events such as PTB; one possible process is chorioamnionitis. Biomarker levels at enrolment, alone or in combination, demonstrated only poor to moderate diagnostic performance.
Low PlGF levels and high sFlt-1:PlGF ratios in third trimester, which are used to predict pre-eclampsia, may be useful to identify women at risk of other adverse pregnancy outcomes36,37. This study finds that lower levels of PlGF at enrolment were associated with SGA, and at delivery, lower PlGF levels and increased sFlt-1:PlGF ratios, together with increased sEng, were associated with LBW and PTB. When stratified by treatment arm, sEng and sFlt-1 elevations at enrolment were associated with PTB only in SPCQ recipients, suggesting that SPAZ abolishes or ablates some pathological angiogenic processes. By contrast, increased sFlt-1:PlGF ratios and increased sFlt-1 were only significantly associated with PTB in SPAZ recipients, possibly reflecting residual disturbances affecting placental function that are independent of infection and inflammation (and thus cannot be prevented/abolished by SPAZ).
The strengths of this study include its setting (low-income, high burden), large sample size, and its part-prospective design and adjustment for confounding factors. To our knowledge this is the first study to assess the impact of antimalarial interventions on biomarker associations with adverse pregnancy outcomes. It allows further insight into the mechanisms of action of SPAZ, contributes to the growing body of evidence of secondary effects of IPTp regimens29, and provides further evidence for the role of inflammation and disturbed placental angiovasculogenesis in causing adverse pregnancy outcomes. Results of this research may be most applicable to LMICs, in particular the role of prophylactic antibiotic therapy, yet further evaluation of anti-inflammatory drugs may also be warranted in high-income settings.
Cross-sectional analysis of associations between biomarker levels at delivery and birth outcomes precludes causal inference. Associations at delivery may be biased as levels of sEng, sFlt-1 and PlGF are known to change in a non-linear fashion with advancing gestational age38. However, CRP levels may be comparatively stable during pregnancy39,40, and the mean difference in gestational age between treatment arms was less than two days. Biomarker levels were only measured twice during pregnancy, limiting longitudinal analysis of changes in biomarker levels during gestation. Statistically significant differences in levels between treatment arms were observed for both AGP and CRP at the 31–35 gestational weeks range (Fig. 3, Supplemental Fig. 1). However, bias is possible as more measurements were obtained from SPCQ recipients during this gestational age range given they were more likely to delivery preterm.
The study has other limitations. First, due to the imprecision associated with estimating gestational age using clinical measures such as last menstrual period in our setting41, PTB and SGA analyses were restricted to ultrasound-dated pregnancies (65% of women), potentially introducing selection bias. Some pregnancies were dated in second trimester, which could have overestimated the incidence of PTB. Second, the low prevalence of HIV infection in our clinics (~1%) precluded assessment of its contribution to adverse outcomes18. Furthermore, infections other than malaria (e.g. sexually transmitted infections, chorioamnionitis) were not assessed in detail to contribute to this analysis. In addition, women randomised to SPAZ had higher CRP levels at antenatal enrolment compared to SPCQ recipients, which may have exaggerated the impact of SPAZ on birth outcomes. Lastly, given the exploratory nature of the research we opted to present p-values that are unadjusted for multiple comparisons. This requires careful interpretation of the data in view of the increased potential for type 1 error.
Limited progress has been made with regards to the prevention of LBW and spontaneous PTB, in particular in LMICs. SPAZ significantly reduces the risk of both LBW and PTB and may do so in part through reducing inflammation and improving placental angiovasculogenesis and function. While the mechanisms by which these agents improve outcomes need definition, further exploration of their therapeutic potential in LMICs is required. The ability of prophylactic antimicrobial therapy in second and third trimester of pregnancy to prevent adverse birth outcomes may depend on the type of antibiotic, dosing regimens, anti-inflammatory properties, and maternal burden of infection and inflammation. Evidence from malaria prevention trials must not be ignored, and further high-quality research from LMICs is required, given the impact such interventions could have in preventing PTB and LBW in these high-burden settings. This includes measuring the impact of IPTp with SPAZ or dihydroartemisinin-piperaquine with AZ on levels of markers of inflammation and angiovasculogenesis assessed longitudinally during pregnancy until delivery.
Materials and Methods
Study design
We analysed samples and data collected as part of a randomised controlled trial of IPTp with SPAZ in PNG (Clinicaltrials.gov, NCT01136850)18. We assessed the relationship between inflammatory/angiogenic marker levels at antenatal enrolment or delivery with birth outcomes in all women and women without malaria, and by treatment arm.
Study site and participants
The study took place from November 2009 until February 2013 at health centres in Madang Province, a malaria-endemic area with a high burden of adverse pregnancy outcomes on the north coast of PNG18. Healthy pregnant women below 26 weeks’ gestation by symphysis fundal height were randomized to either three courses of SP (3 tablets [500/25 mg] given once) and AZ (2 tablets [500 mg] twice daily for 2 days), given at minimum intervals of 4 weeks; or a clearance course of SP and chloroquine (3 or 4 tablets [150 mg], daily for 3 days) at enrolment, followed by monthly courses of placebo equivalent. Women received insecticide-treated bed nets and iron-folate supplementation. Biomarker studies were undertaken amongst women who completed follow-up for birthweight and who gave birth to a congenitally normal singleton infant ≥22 gestational weeks (or ≥500 g if not ultrasound-dated)42. Gestational age at delivery was estimated by ultrasound using the earliest scan available to date the pregnancy18. Analyses evaluating the outcome measures PTB and SGA were restricted to women with ultrasound-dated pregnancies (1,314 of 2,012 women [65.3%]), given clinical estimates of gestational age are prone to significant imprecision in our setting41.
Laboratory analyses
We measured the acute-phase proteins C-reactive protein (CRP) and α-1-Acid glycoprotein (AGP), and angiogenic factors soluble fms-like tyrosine kinase (sFlt-1), PlGF and sEng on stored plasma (thawed from −80 °C) of maternal venous blood samples from enrolment and delivery14. We additionally calculated sFlt-1:PlGF ratios43.
We used commercially available enzyme-linked immunosorbent assays (ELISA) (Human Quantikine ELISA kits; R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions. Human plasma samples were diluted with BSA-PBS (sEng: 1:25, PIGF and sFlt1: 1:5; CRP: 1:10,000; AGP: 1:1,000,000) and assayed in duplicates. Optical densities were read at 450 nm and 540 nm on a Fluostar Omega microplate reader (BMG Labtech, Ortenberg, Germany). Sample concentrations were interpolated from a standard curve and multiplied by the dilution factor. Samples above or below the standard curve, or with duplicates varying by >20%, were repeated, with dilution adjustments if required.
Statistical analysis
Univariate comparisons of categorical data were performed using the Chi-squared test or Fisher’s exact test, as appropriate. Univariate comparisons of continuous parametric data (including normalised data) were performed using the Student’s t-test, and non-parametric data using the Mann Whitney-U test or Wilcoxon signed-rank test, as appropriate.
Logistic regression analyses were performed to evaluate associations between biomarker levels at enrolment or delivery and adverse birth outcomes (PTB, SGA, LBW). Biomarker distribution exhibited a strong positive skew. Biomarker data were log(10) transformed to normalise the data prior to inclusion in the model as a continuous predictor44. Biomarker levels were presented as geometric means (95% confidence intervals). P < 0.05 was considered significant.
SGA was defined as a birthweight below the 10th centile of the Intergrowth-2145. Models were adjusted a priori for factors previously found to be associated with LBW in the cohort (bed net use, mid-upper arm circumference, recruitment clinic, maternal height, socio-economic status, number of antenatal visits, sex of the baby, timing of birthweight measurement)18. We additionally adjusted for gestational age at enrolment to account for fluctuations in biomarker levels during pregnancy38,46. Associations between biomarkers and outcomes were assessed overall, and by trial treatment arm. When using birthweight as the outcome measure, biomarker levels were included in the linear regression model as dichotomised variables: a raised CRP was defined as a CRP ≥ 5 mg/L47, elevated levels of AGP, sFlt-1 and sEng were defined as readings in the 4th quartile, and a low PlGF as the first quartile. A sFlt-1:PlGF ratio ≥ 38 was defined as elevated43. We additionally evaluated combinations of CRP and AGP levels, given their differing temporal dynamics48.
General additive models were fitted to the longitudinal data of biomarkers measured at enrolment and birth and gestational age, separately for SPAZ and SPCQ treatment arms. Curve fitting was conducted using R and its ‘gam’ package. Generalised additive models were allowed 4 degrees of freedom. Multilevel linear mixed effects models (Stata 13) were used to assess changes in biomarker levels with gestational age and to evaluate whether there were any differences in the biomarker level-gestational age relationship between treatment arms.
Because SPAZ significantly reduced peripheral and placental blood parasitemia and active malaria infection on placental histology18, and active placental infection was associated with LBW and PTB in the cohort49, we investigated whether associations between biomarkers and adverse outcomes according to treatment arm persisted in women unlikely to have had malaria. Specifically, we excluded all women who at least once tested positive during pregnancy for P. falciparum or P. vivax on peripheral or placental blood smear, and/or polymerase chain reaction of peripheral and placental blood, or when available, placental histology (active infection)16,49. Peripheral blood testing was performed at enrolment, at subsequent IPTp visits, and at delivery18.
Diagnostic characteristics of biomarkers at enrolment to predict LBW, PTB, and SGA were assessed amongst women subsequently randomised to SPCQ and included receiver-operator curve characteristics (area under the curve [AUC]), the Youden index, sensitivity, specificity, positive and negative predictive values and positive and negative likelihood ratios.
Ethical considerations
Ethical approval for this study was obtained from the PNG Institute of Medical Research (PNGIMR) Institutional Review Board, the PNG Medical Research Advisory Council, and the Melbourne Health Human Research Ethics Committee (Australia). The study was conducted in accordance with Good Clinical Practice guidelines (ICH GCP E6). All participants provided written informed consent.
Supplementary information
Acknowledgements
We would like to thank all participants, their families and children; Dr. Alex Umbers, Dr. Leanne Robinson, and Dr. Regina Wangnapi; and all staff at the PNG Institute of Medical Research, without whom the study would not have been possible. The work was funded by a Thrasher Early Career Award (12934 to H.W.U); the Malaria in Pregnancy Consortium, through a grant from the Bill & Melinda Gates Foundation (46099 to S.J.R); Pregvax Consortium, through a grant from the European Union’s Seventh Framework Programme (FP7-2007-HEALTH, PREGVAX 201588 to S.J.R and I.M.) and the Spanish Government (EUROSALUD 2008 Programme); a Pfizer Inc Investigator-Initiated Research grant (WS394663 to S.J.R); an National Health and Medical Research (NHMRC) Program Grant (1092789 to S.J.R, J.G.B, I.M.); NHMRC Fellowships (1077636 to J.G.B, 1043345 to I.M., GNT1141441 to S.K.); a Wellcome Trust Clinical Career Development Fellowship (209560/Z/17/Z to S.J.S); and Tommy’s Charity (to S.J.S). The funding bodies had no role in study design, and the collection, analysis and interpretation of the data.
Author Contributions
The study was conceived and designed by S.J.R., H.W.U. and S.J.S. Data collection was supervised by H.W.U., M.O.K., I.M. and S.J.R. Data management and cleaning was undertaken by H.W.U. and M.O.K. Laboratory analyses were conducted and overseen by A.P.H., C.B., W.H., A.T., A.A.A., L.R. and S.J.R. Data analysis was undertaken by H.W.U. and S.K. The report was written by H.W.U. and S.J.R., with substantial input from J.G.B. and S.J.S. All authors have read and commented on the final manuscript.
Data Availability
Parent trial data are available from the WWARN data repository (http://www.wwarn.org/working-together/sharing-data/accessing-data) for researchers who meet the criteria for access to confidential data. Biomarker data are available from the corresponding author by written request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38821-2.
References
- 1.Harrison MS, Goldenberg RL. Global burden of prematurity. Seminars Fetal & Neonatal Med. 2016;21:74–79. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Chico RM, et al. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA. 2012;307:2079–2086. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 3.Vogel JP, Lee AC, Souza JP. Maternal morbidity and preterm birth in 22 low- and middle-income countries: a secondary analysis of the WHO Global Survey dataset. BMC Pregnancy Childbirth. 2014;14:56. doi: 10.1186/1471-2393-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 5.Marchant T, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9:e1001292. doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ernst GD, et al. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. Am. J. Obstet. Gynecol. 2011;205:132.e1–12. doi: 10.1016/j.ajog.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Rebelo F, et al. C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status. J. Hypertens. 2013;31:16–26. doi: 10.1097/HJH.0b013e32835b0556. [DOI] [PubMed] [Google Scholar]
- 8.Sorokin Y, et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am. J. Perinatol. 2010;27:631–640. doi: 10.1055/s-0030-1249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy AL, Liles WC, Molyneux ME, Rogerson SJ, Kain KC. Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi. PLoS One. 2012;6:e28540. doi: 10.1371/journal.pone.0028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton AL, et al. Periodontal disease early in pregnancy is associated with maternal systemic inflammation among African American women. J. Periodontol. 2008;79:1127–1132. doi: 10.1902/jop.2008.070655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karinen L, et al. Serum C-reactive protein and Chlamydia trachomatis antibodies in preterm delivery. Obstet. Gynecol . 2005;106:73–80. doi: 10.1097/01.AOG.0000164464.11979.5d. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Conroy AL, et al. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe. 2013;13:215–226. doi: 10.1016/j.chom.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Radeva-Petrova D, Kayentao K, Ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst. Rev. 2014;10:CD000169. doi: 10.1002/14651858.CD000169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umbers AJ, et al. Accuracy of an HRP-2/panLDH rapid diagnostic test to detect peripheral and placental Plasmodium falciparum infection in Papua New Guinean women with anaemia or suspected malaria. Malar. J. 2015;14:412. doi: 10.1186/s12936-015-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogerson SJ, Unger HW. Prevention and control of malaria in pregnancy - new threats, new opportunities? Expert Rev. Anti. Infect. Ther. 2016;15:361–375. doi: 10.1080/14787210.2017.1272411. [DOI] [PubMed] [Google Scholar]
- 18.Unger HW, et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Med. 2015;13:9. doi: 10.1186/s12916-014-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luntamo M, et al. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. Am. J. Trop. Med. Hyg. 2010;83:1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chico RM, Hack BB, Newport MJ, Ngulube E, Chandramohan D. On the pathway to better birth outcomes? A systematic review of azithromycin and curable sexually transmitted infections. Expert Rev. Anti. Infect. Ther. 2013;11:1303–1332. doi: 10.1586/14787210.2013.851601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson DW, et al. Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 2015;13:52. doi: 10.1186/s12915-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratjen F, et al. Effect of azithromycin on systemic markers of inflammation in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa. Chest. 2012;142:1259–1266. doi: 10.1378/chest.12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chico RM, Chaponda EB, Ariti C, Chandramohan D. Sulfadoxine-Pyrimethamine Exhibits Dose-Response Protection Against Adverse Birth Outcomes Related to Malaria and Sexually Transmitted and Reproductive Tract Infections. Clin. Infect. Dis. 2017;64:1043–1051. doi: 10.1093/cid/cix026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoner MC, et al. Dosage of Sulfadoxine-Pyrimethamine and Risk of Low Birth Weight in a Cohort of Zambian Pregnant Women in a Low Malaria Prevalence Region. Am. J. Trop. Med. Hyg. 2017;96:170–177. doi: 10.4269/ajtmh.16-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai M, et al. Impact of Sulfadoxine-Pyrimethamine Resistance on Effectiveness of Intermittent Preventive Therapy for Malaria in Pregnancy at Clearing Infections and Preventing Low Birth Weight. Clin. Infect. Dis. 2016;62:323–333. doi: 10.1093/cid/civ881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanisic DI, et al. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 2015;109:313–324. doi: 10.1093/trstmh/trv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wangnapi RA, et al. Prevalence and risk factors for Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis infection in pregnant women in Papua New Guinea. Sex. Transm. Infect. 2014;91:194–200. doi: 10.1136/sextrans-2014-051670. [DOI] [PubMed] [Google Scholar]
- 28.Fowkes FJI, et al. Iron deficiency during pregnancy is associated with a reduced risk of adverse birth outcomes in a malaria-endemic area in a longitudinal cohort study. BMC Med. 2018;16:156. doi: 10.1186/s12916-018-1146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger HW, et al. Azithromycin-containing intermittent preventive treatment in pregnancy affects gestational weight gain, an important predictor of birthweight in Papua New Guinea - an exploratory analysis. Matern. Child. Nutr. 2016;12:699–712. doi: 10.1111/mcn.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harjunmaa U, et al. Association between maternal dental periapical infections and pregnancy outcomes: results from a cross-sectional study in Malawi. Trop. Med. Int. Health. 2015;20:1549–1558. doi: 10.1111/tmi.12579. [DOI] [PubMed] [Google Scholar]
- 31.Callahan BJ, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA. 2017;114:9966–9971. doi: 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, et al. Prevalence and Clinical Significance of Sterile Intra-amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am. J. Reprod. Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst. Rev. 2015;6:CD002250. doi: 10.1002/14651858.CD002250.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Broek NR, et al. The APPLe study: a randomized, community-based, placebo-controlled trial of azithromycin for the prevention of preterm birth, with meta-analysis. PLoS Med. 2009;6:e1000191. doi: 10.1371/journal.pmed.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 36.Griffin M, et al. Diagnostic accuracy of placental growth factor and ultrasound parameters to predict the small-for-gestational-age infant in women presenting with reduced symphysis-fundus height. Ultrasound Obstet. Gynecol. 2015;46:182–190. doi: 10.1002/uog.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins L, Heazell A, Johnstone E, Myers J. A high third trimester sFlt1:PlGF ratio is associated with reduced fetal growth velocity (PP.016) BJOG. 2018;125(Suppl 2: 4111):91–92. [Google Scholar]
- 38.Conroy AL, et al. Altered angiogenesis as a common mechanism underlying preterm birth, small for gestational age, and stillbirth in women living with HIV. Am. J. Obstet. Gynecol. 2017;217:684.e1–14. doi: 10.1016/j.ajog.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet. Gynecol. 1991;77:176–180. doi: 10.1097/00006250-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, et al. Metabolic profiling of pregnancy: cross-sectional and longitudinal evidence. BMC Med. 2016;14:205. doi: 10.1186/s12916-016-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karl, S. et al. Preterm or not - an evaluation of estimates of gestational age in a cohort of women from rural papua new Guinea. PLoS One10 (2015). [DOI] [PMC free article] [PubMed]
- 42.Rijken MJ, et al. Malaria in pregnancy: the difficulties in measuring birthweight. BJOG. 2011;118:671–678. doi: 10.1111/j.1471-0528.2010.02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeisler H, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 44.Collins GS, Ogundimu EO, Cook JA, Manach YL, Altman DG. Quantifying the impact of different approaches for handling continuous predictors on the performance of a prognostic model. Stat. Med. 2016;35:4124–4135. doi: 10.1002/sim.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villar J, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 46.Darling AM, et al. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. Am. J. Obstet. Gynecol. 2014;211:509. e1–8. doi: 10.1016/j.ajog.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurnham DI, et al. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J. Clin. Nutr. 2010;92:546–555. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 48.Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J. Nutr. 2015;145:1137S–1143S. doi: 10.3945/jn.114.194712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lufele E, et al. Risk factors and pregnancy outcomes associated with placental malaria in a prospective cohort of Papua New Guinean women. Malar. J. 2017;16:427. doi: 10.1186/s12936-017-2077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Parent trial data are available from the WWARN data repository (http://www.wwarn.org/working-together/sharing-data/accessing-data) for researchers who meet the criteria for access to confidential data. Biomarker data are available from the corresponding author by written request.