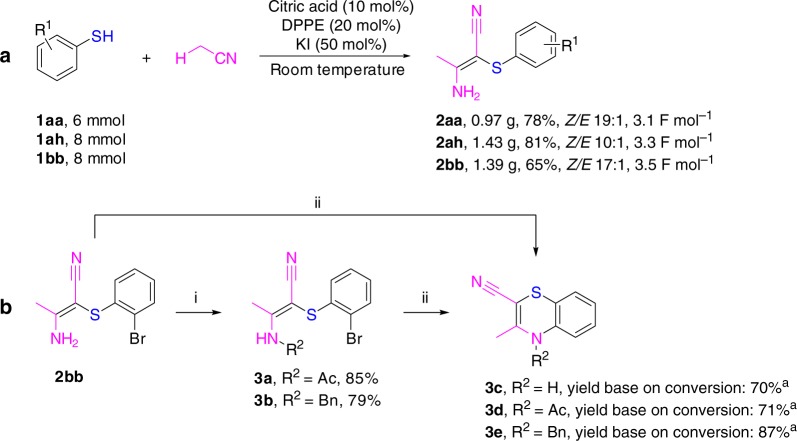
Fig. 3.
Gram-scale synthesis and product transformations. Reaction conditions: (i) acetyl chloride, Et3N, CH2Cl2, 0 °C to reflux, 12 h, 85%; BnBr, NaH, dry N,N-dimethylformamide (DMF), N2, 0 °C to r.t., 4 h, 79%. (ii) CuI, K2CO3, trans-N,N′-dimethylcyclohexane-1,2-diamine, N,N′-dimethylethylenediamine, toluene, N2, 120 °C, 15 h, conditions to be optimized. aConversions: 3c, 60%; 3d, 72%; 3e, 62%