FIGURE 1.
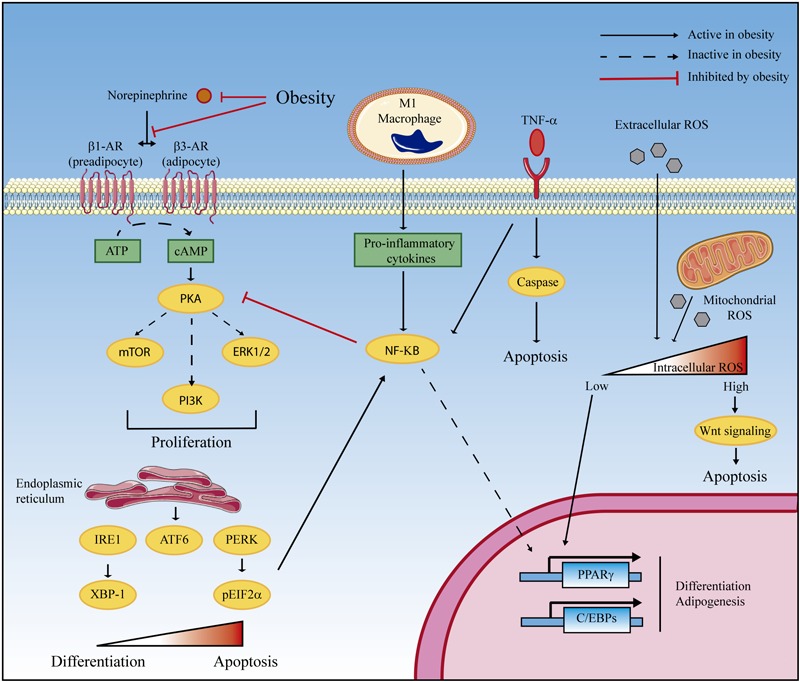
Proposed mechanisms of obesity-induced BAT depletion. BAT mass and activity are minimized in obese patients due to reduced cell proliferation and preadipocyte differentiation and increased apoptosis. At least four obesity-related mechanisms can be involved. (1) Catecholamine resistance in obesity is characterized by decreased synthesis of norepinephrine and beta-adrenergic (β-AR) receptors and by defective intracellular signaling, which impedes PKA-mediated cell proliferation. (2) Obesity promotes the infiltration of M1 macrophages that participate in norepinephrine clearance and contribute to the synthesis of proinflammatory cytokines. NF-κB-mediated signaling inhibits the PKA proliferation pathway and represses PPARγ and C/EBPs gene expression, which inhibits differentiation and adipogenesis. In addition, TNF-α overexpression triggers cellular apoptosis. (3) The unfolded protein response (UPR) in the endoplasmic reticulum plays a dual role in brown adipocyte differentiation according to the intensity of the signal. While activation of the three branches of UPR (IRE-1, ATF6, and PERK) is required for differentiation, excessive UPR activation (that can be found in severe obesity) triggers proapoptotic mechanisms. (4) In a similar manner, reactive oxygen species (ROS) are also hormetic regulators of cell differentiation and apoptosis. Physiological ROS concentrations promote C/EBP expression leading to differentiation, whereas supraphysiological concentration leads to oxidative stress and apoptosis via Wnt signaling. Artwork was obtained from Servier Medical Art, licensed under a Creative Common Attribution 3.0 Generic License (http://smart.servier.com/).
