Although autism spectrum disorder (ASD) is recognized as having a strong genetic component, a cause cannot be identified for most affected individuals, reflecting the complexity of underlying genetic mechanisms and possible environmental interactions.1,2 Researchers have examined potential biomarkers, such as carnitine (3-hydroxy-4-N-trimethylaminobutyrate), which is proposed to be a conditionally essential nutrient,3 with its primary role being to transport long-chain fatty acids from the cytosol to the mitochondrial matrix, where they undergo β-oxidation to produce energy. Carnitine homeostasis is maintained by dietary absorption, a modest rate of endogenous biosynthesis, and an efficient renal reabsorption. Carnitine administration is beneficial in multiple causes of carnitine deficiency4 and genetic conditions such as Rett syndrome.5 High doses of carnitine up to 400 mg/kg/day are used to treat systemic carnitine deficiency and are believed to be safe, with occurrence of mild diarrhea and an unusual odor being the only observed side effect.6
Current Study: Carnitine Biosynthetic Pathway Defects and ASD
Detection of a deletion in the TMLHE gene, encoding trimethyllysine hydroxylase epsilon, in an individual with ASD led to the delineation of a novel inborn error of carnitine biosynthesis.7 Subsequently, other TMLHE mutations identified in children with ASD raised the possibility of a relation between carnitine biosynthetic pathway defects and autism,8,9 leading to the hypothesis that brain carnitine deficiency may cause some cases of ASD.10 Males born with the deficiency have a 3% chance of being diagnosed with ASD—2-fold higher than the general population’s risk for males. A prospective double-blind, randomized clinical trial of levocarnitine to treat ASD found that levocarnitine therapy (50 mg/kg-body weight/day) for 3 months decreased ASD severity per scores on the Childhood Autism Rating Scale (CARS)11 and the Autism Treatment Evaluation Checklist (ATEC).12,13 However, the authors felt that levocarnitine dosage may have been too low and that greater clinical improvements could be observed at higher doses.
We conducted an open-label, pilot trial among young males with ASD to examine dose compliance, attrition, and potential side effects of short-term, high-dose carnitine supplementation. Secondary outcomes focused on possible (1) behavioral improvements following carnitine supplementation and (2) biochemical-behavioral relationships between free carnitine levels in plasma and select behavioral indices.
Methods
Participants
Participants were 10 males (mean age = 59.8 months [5.0 years], SD = 22.7 months [1.9 years], range = 31-92 months [2.7-7.7 years]) who were recruited from local samples of participants from the Simons Simplex Collection (SSC)14 or the Autism Treatment Network. Six identified as Caucasian, 2 as Hispanic, 1 as biracial Caucasian/African American, and 1 as Asian Indian. One child had documented TMLHE deficiency and 3 had low carnitine levels (see Table 1 for details). Children were not asked to stop/withhold any therapies that they were pursuing at enrollment. None were taking medications that were contraindicated for high-dose carnitine supplementation.
Table 1.
Demographic Characteristics, Plasma Carnitine Levels, Carnitine Dose Titration, Side Effects, and Behavioral Outcomes.
| ID | Agea | ADI-R S/C/Rb | VMA/NVMAc | Dev Regd | Plasma Carnitine (in µM), Pre/Poste | Study Status | Dose Titration | Side Effect(s) | CGISf |
Anecdotal Parent Reports | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity, Pre/Postg | GIh | EIi | ||||||||||
| 1 | 81 | 18/19V/3 | 32/43 | No | 37.3/34.9 | Early termination after 17 days | Unable to tolerate up-titration | Heavy odor | 4/4 | 3 | Unchanged or worse therapeutic effect + side effects do not significantly interfere with patient’s functioning | Child exhibited some hyperactivity at the start of the study, but parents felt they may be unrelated to treatment. There were no noticeable changes reported aside from heavy odor. Parents chose to discontinue supplementation at the start of school (after 4 weeks). |
| 2 | 82 | 23/22V/10 | 101/133 | No | 39.2/81.1 | Completed study | Compliant with dose schedule | Rash unrelated to study; decreased sensitivity in fingers; slight odor | 3/2 | 3 | Slight improvement, which does not alter status of care of patient + no side effects | Mother was happy with his behavior on treatment (eg, calmer, happier at school). He had more energy by the end of the study. No major changes were noted. |
| 3 | 72 | — | 37/46 | — | 40.3/— | Early termination | Compliant with dose schedule | Did not tolerate solution and was switched to tablets with no further side effects | 5/— | — | — | Child had negative behaviors prior to study participation, and parents did not report appreciable changes while on treatment. Family was lost to follow-up after mid-treatment. |
| 4 | 47 | 13/13NV/5 | 9/11 | Yes | 23.4/40.9 | Completed study | Compliant with dose schedule | Heavy odor; some vomiting episodes | 6/4 | 2 | Decided improvement with partial remission of symptoms + side effects significantly interfere with patient’s functioning | Child exhibited more socially acceptable behavior by day 11 on treatment; he was more aware of his surroundings and had appropriate responses by day 29. Despite reported behavioral improvements while on treatment, parents discontinued supplementation because of sporadic vomiting episodes. |
| 5 | 70 | 29/13NV/5 | 4/19 | Yes | 33/36 | Completed study | Compliant with dose schedule | None reported | 6/6 | 4 | Unchanged or worse therapeutic effect + no side effects | Child was more interactive and calmer by day 7 on treatment, but no other major behavioral changes were noted by parents. |
| 6 | 80 | 17/7NV/4 | 45/46 | No | 38.9/46.1 | Completed study | Unable to tolerate up-titration | Mild diarrhea at start of study | 5/4 | 3 | Slight improvement which does not alter status of care of patient + no side effects | Child exhibited subtle, positive behavioral changes and increased energy. |
| 7 | 34 | 9/11V/9 | 37/28 | Yes | 24/121 | Completed study | Compliant with dose schedule | Mild diarrhea throughout study | 4/3 | 3 | Slight improvement which does not alter status of care of patient + side effects do not significantly interfere with patient’s functioning | Child was irritable at the beginning of the study. Mother reported improved eye contact while on treatment. Child reportedly maintained increased gestures, eye contact, and directed communications. |
| 8 | 33 | 21/10NV/4 | 11/21 | Yes | 30/51 | Completed study | Unable to tolerate up-titration | Some diarrhea noted on a few days; sleep-schedule changes | 6/5 | 4 | Unchanged or worse therapeutic effect + side effects significantly interfere with patient’s functioning | Child experienced sleep disturbances (eg, woke up at night and would not fall back to sleep). Child also reportedly experienced episodes of increased energy and stemming at the beginning of the study. He had increased awareness and expanded toy play by day 52. |
| 9 | 34 | 9/13V/7 | 28/28 | Yes | 28/118.3 | Completed study | Compliant with dose schedule | Heavy odor | 4/3 | 2 | Decided improvement with partial remission of symptoms + side effects do not significantly interfere with patient’s functioning | Child was reportedly more attentive and exhibited appropriate social behavior and less aggression during treatment. Improvements in language skills were also noted. |
| 10 | 48 | 26/13NV/7 | 22/27 | Yes | 28/70 | Completed study | Compliant with dose schedule | Loose bowel movements | 5/5 | 4 | Unchanged or worse therapeutic effect + side effects do not significantly interfere with patient’s functioning | Child was lethargic at the beginning of the study. He exhibited aggressive behavior when on high-dose carnitine supplementation. Stemming behaviors were reduced and energy level was low, but the latter did not worsen as the study progressed. Child was reportedly more social and exhibited more eye contact. |
Age at baseline in months.
ADI-R S/C/R = Autism Diagnostic Interview—Revised (ADI-R) domain total scores for reciprocal social interaction (S)/Qualitative Abnormalities in Communication (C; Verbal [V] or Nonverbal [NV])/restricted, repetitive, and stereotyped patterns of behavior (R).
VMA/NVMA = verbal mental age/nonverbal mental age.
Dev Reg = history of language and/or other type of developmental regression per the ADI-R.
Pre/Post = carnitine levels in plasma at baseline/post treatment.
CGIS = Clinical Global Impression Scale.
Severity Pre/Post = rating of severity of illness (ASD) at baseline/post treatment per the CGIS; 2 = borderline mentally ill; 3 = mildly mentally ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill.
GI = rating of global improvement per the CGIS; 2 = much improved; 3 = minimally improved; 4 = no change.
EI = Efficacy Index per the CGIS.
Ethical Approval and Informed Consent
This study was approved by the Institutional Review Board at Baylor College of Medicine, and all participants provided written consent to cooperate with study procedures.
Instruments
Developmental/Cognitive Functioning
Children’s developmental/cognitive abilities were measured at baseline with either the Mullen Scales of Early Learning15 (n = 7) or the Differential Ability Scales, Second Edition (DAS-II16; n for Early Years version = 2, n for School Age version = 1). Composite scores of verbal mental age, nonverbal mental age, and full-scale mental age were compared.
ASD Symptomatology
Parent-reported information about children’s ASD behaviors was gathered via the Autism Diagnostic Interview–Revised (ADI-R).17 If a previous, research-reliable ADI-R was completed and available to the study team, those data were used. Current, clinician-observed ASD behaviors were assessed via the Autism Diagnostic Observation Schedule (ADOS).18 While the original ADOS was administered during the current study, subscale scores were calculated using the updated ADOS-219 algorithm. If a research-reliable ADOS was completed within 6 months of enrollment for the current study, then those data were used at baseline.
Parent Report of Behavior Over Time
Parent ratings of current ASD behaviors were assessed using the Social Communication Questionnaire–Current version (SCQ),20 the Pervasive Developmental Disorder Behavior Inventory (PDDBI),21 and the Autism Impact Measure (AIM).22 Problematic behaviors were assessed using the Aberrant Behavior Checklist (ABC).23
Clinician-Rated Improvement/Efficacy
Study examiners completed the Clinical Global Impression Scale (CGIS [Early Clinical Drug Evaluation Program version24]) to capture their impressions of potential behavior changes.
Procedures
Consenting families participated in an 8-week, open-label trial of carnitine supplementation. Families were advised to report immediately any unusual behavior or adverse events and to keep a drug diary to record any doses missed during the trial.
Behavioral assessments and biochemical assays were collected at 3 time points: baseline (0 weeks), mid-treatment (4 weeks), and post treatment (8 weeks). Logistic and scheduling issues delayed posttreatment data collection for 4 cases beyond the planned 8-week window; however, all remained on carnitine supplementation until their final blood draw.
Medical Examination and Sample Collection
A baseline physical examination was performed, and a medical history was taken to screen for medical conditions affected by high-dose carnitine supplements (eg, gastroenteritis). Blood and urine samples were taken at up to 3 different time points (baseline, mid-treatment, post treatment) to measure plasma carnitine and related metabolites, determine TMLHE deficiency, and screen for renal and liver function. Mid-treatment samples were not taken for 6 children who could not return to the study site. Safety laboratories were performed, and the final results were shared with the study team. The research blood samples were sent to the Beaudet Laboratory at Baylor College of Medicine.
Behavioral Assessments
At baseline, all children participated in cognitive testing with either the Mullen or the DAS-II; the ADOS and/or ADI-R were also administered to confirm ASD status. The ADOS was repeated at post treatment. All 10 participants had complete baseline data for the cognitive assessments and ADOS, and all but one had complete data for the ADI-R. Parents completed the SCQ, PDDBI, AIM, and ABC at baseline, mid-treatment, and post treatment to measure more subtle behavioral changes over time. Clinicians completed the CGIS at baseline and post treatment, with reports informed by their interactions with children during standardized assessments (ie, cognitive testing/ADOS at baseline, ADOS at post treatment).
Carnitine Supplementation
Participating children were administered oral suspension or tablets of levocarnitine in 3 divided doses, starting at 200 mg/kg/day and increasing to 400 mg/kg/day, with a maximum daily dose of 6 g. If a child experienced unpleasant side effects (ie, fishy body/breath odor, diarrhea), the maximum daily dose was dropped down to 200 mg/kg/day. Fisherman’s soap and probiotics were recommended to further alleviate unpleasant side effects.
Statistical Analyses
Descriptive statistics were employed to characterize the sample demographically and phenotypically. Primary outcomes of interest were dose compliance, side effects, and attrition. Because this was an exploratory pilot trial, all subscale and total scores for the aforementioned measures were analyzed as secondary outcome variables. A random coefficient model was used to estimate the effect of time on carnitine supplementation on each outcome variable. The model included fixed effects for time and random coefficients for the intercept and time. Because of convergence problems (ie, small sample size), the model for carnitine level in plasma only included a random slope, and the model for the AIM’s Social-Emotional Reciprocity Frequency ratings only included a random intercept. Spearman’s rank correlation statistic was used to estimate the correlation between carnitine levels and outcome measures by visit number (baseline = 1, mid-treatment = 2, post treatment = 3). Results from the CGIS were analyzed descriptively.
Results
The following side effects were reported: heavy odor (4 parents), diarrhea (4 parents), and sporadic vomiting (1 parent). The one child who experienced sporadic vomiting had a disruption in supplementation (ie, was off treatment for 7 days). Dose compliance was otherwise good, which was verified by increased levels of plasma carnitine from pre- to post-assessment. Three children could not tolerate up-titration because of increased side effects (odor, diarrhea). These children remained at the lower dosage, with one discontinuing supplementation by mid-treatment because odor remained too problematic for the family. Three parents indicated no observable side effects; one of these families was lost to follow-up after the mid-treatment assessment. Favorable anecdotes reported by families included the following: calmer behavior (2 parents), more energy (2 parents), increased prosocial behaviors (4 parents), greater awareness (2 parents), better eye contact (2 parents), and improved language skills (2 parents). A complete listing of dose titration, side effects, and anecdotal reports are presented in Table 1.
Scores on the ABC Hyperactivity subscale, SCQ, PDDBI Social Pragmatic Problems domain, and AIM Social-Emotional Reciprocity Impact domain significantly decreased over time (indicating behavioral improvement) at the unadjusted .05 level. Similarly, PDDBI Social Approach Behaviors, PDDBI Expressive Social Communication Abilities Composite scores, and PDDBI Receptive/Expressive Social Communication Abilities Composite scores significantly increased over time (indicating behavioral improvement). However, there were no significant changes in any scores over time after adjusting for multiple comparisons using Holm’s step-down Bonferroni correction (P ≥ .31; see Table 2).
Table 2.
Estimates for Changes in Behavioral Indices Over Time.
| Outcome Variable | Estimate | Standard Error | Degrees of Freedom | t | P |
|---|---|---|---|---|---|
| Carnitine levels | 0.548 | 0.191 | 10.3 | 2.86 | .016 |
| ABC Irritability | −0.088 | 0.066 | 7.9 | −1.32 | .223 |
| ABC Lethargy | −0.075 | 0.052 | 6.8 | −1.44 | .196 |
| ABC Stereotypy | −0.045 | 0.025 | 7.3 | −1.85 | .106 |
| ABC Hyperactivity | −0.155 | 0.060 | 8.9 | −2.58 | .030 |
| ABC Speech | −0.014 | 0.020 | 6.1 | −0.71 | .505 |
| ADOS SA | −0.008 | 0.017 | 8.6 | −0.45 | .663 |
| ADOS RRB | −0.001 | 0.011 | 8.3 | −0.13 | .897 |
| ADOS Total | −0.007 | 0.019 | 8.8 | −0.37 | .721 |
| SCQ Total | −0.071 | 0.028 | 6.2 | −2.55 | .043 |
| PDDBI Sensory | −0.068 | 0.047 | 8.9 | −1.46 | .179 |
| PDDBI Ritual | −0.015 | 0.087 | 8.1 | −0.18 | .863 |
| PDDBI SOCPP | −0.110 | 0.040 | 14 | −2.73 | .017 |
| PDDBI SEMPP | −0.085 | 0.063 | 7.4 | −1.36 | .212 |
| PDDBI Arouse | −0.132 | 0.067 | 8.9 | −1.97 | .081 |
| PDDBI Fears | −0.065 | 0.043 | 13 | −1.51 | .154 |
| PDDBI AGG | 0.007 | 0.115 | 8.2 | 0.06 | .956 |
| PDDBI REPRIT/C | −0.088 | 0.063 | 8.3 | −1.40 | .198 |
| PDDBI AWP/C | −0.099 | 0.070 | 8.5 | −1.41 | .193 |
| PDDBI SOCAPP | 0.108 | 0.027 | 5.4 | 3.96 | .009 |
| PDDBI Express | 0.010 | 0.023 | 8.6 | 0.41 | .693 |
| PDDBI LMRL | 0.006 | 0.034 | 4.5 | 0.18 | .862 |
| PDDBI EXSCA/C | 0.057 | 0.020 | 6.6 | 2.90 | .025 |
| PDDBI REXSCA/C | 0.054 | 0.022 | 7.7 | 2.42 | .043 |
| PDDBI Autism | −0.116 | 0.060 | 8.3 | −1.92 | .090 |
| AIM RRB-F | −0.034 | 0.076 | 4.5 | −0.44 | .681 |
| AIM RRB-I | −0.069 | 0.039 | 7 | −1.76 | .123 |
| AIM COMM-F | −0.022 | 0.021 | 13 | −1.08 | .301 |
| AIM COMM-I | −0.015 | 0.021 | 13 | −0.72 | .487 |
| AIM SER-F | −0.038 | 0.022 | 13.3 | −1.76 | .1013 |
| AIM SER-I | −0.060 | 0.026 | 13 | −2.31 | .038 |
| AIM OAB-F | −0.036 | 0.022 | 7.6 | −1.65 | .140 |
| AIM OAB-I | −0.031 | 0.020 | 8 | −1.57 | .156 |
Abbreviations: ABC, Aberrant Behavior Checklist; ADOS, Autism Diagnostic Observation Schedule; ADOS SA, ADOS Social Affect; ADOS RRB, ADOS Restricted and Repetitive Behavior; SCQ, Social Communication Questionnaire; PDDBI, Pervasive Developmental Disorder Behavior Inventory; PDDBI Sensory, PDDBI Sensory/Perceptual Approach Behaviors; PDDBI Ritual, PDDBI Ritualisms/Resistance to Change; PDDBI SOCPP, PDDBI Social Pragmatic Problems; PDDBI SEMPP, PDDBI Semantic/Pragmatic Problems; PDDBI Arouse, PDDBI Arousal Regulation Problems; PDDBI Fears, PDDBI Specific Fears; PDDBI AGG, PDDBI Aggressiveness; PDDBI REPRIT/C, PDDBI Repetitive, Ritualistic, and Pragmatic Problems Composite; PDDBI AWP/C, PDDBI Approach/Withdrawal Problems Composite; PDDBI SOCAPP, PDDBI Social Approach Behaviors; PDDBI Express, PDDBI Expressive Language; PDDBI LMRL, PDDBI Learning, Memory, and Receptive Language; PDDBI EXSCA/C, PDDBI Expressive Social Communication Abilities Composite; PDDBI REXSCA/C, PDDBI Receptive/Expressive Social Communication Abilities Composite; PDDBI Autism, PDDBI Autism Composite; AIM, Autism Impact Measure; AIM RRB-F, AIM Restricted/Repetitive Behavior Frequency; AIM RRB-I = AIM Restricted/Repetitive Behavior Impact; AIM COMM-F, AIM Communication/Language Frequency; AIM COMM-I, AIM Communication/Language Impact; AIM SER-F, AIM Social-Emotional Reciprocity Frequency; AIM SER-I, AIM Social-Emotional Reciprocity Impact; AIM OAB-F, AIM Odd/Atypical Behavior Frequency; AIM OAB-I, AIM Odd/Atypical Behavior Impact. P-values in bold reflect those that are significant at less than .05.
Among all carnitine-outcome pairs for which there were at least 7 observations available, plasma carnitine levels were correlated with a number of PDDBI subscale scores, as well as the ABC Irritability subscale. Only the PDDBI Learning, Memory, and Receptive Language domain maintained statistical significance at the .05 level after adjusting for multiple comparisons (P = .04). All of these correlations surfaced only at visit 3 (see Table 3).
Table 3.
Correlations Between Select Behavioral Indices and Posttreatment Carnitine Levelsa.
| Variable | r With Carnitine Levels | P | Adjusted P |
|---|---|---|---|
| PDDBI LMRL | 0.964 | <.001 | .039 |
| PDDBI Express | 0.955 | <.001 | .069 |
| PDDBI EXSCA/C | 0.865 | .012 | 1.000 |
| PDDBI REXSCA/C | 0.865 | .012 | 1.000 |
| PDDBI Ritual | 0.811 | .027 | 1.000 |
| ABC Irritability Subscale | 0.739 | .058 | 1.000 |
| PDDBI SEMPP | 0.714 | .071 | 1.000 |
| PDDBI AGG | 0.679 | .094 | 1.000 |
| PDDBI SOCAPP | 0.679 | .094 | 1.000 |
Abbreviations: PDDBI, Pervasive Developmental Disorder Behavior Inventory; PDDBI LMRL, PDDBI Learning, Memory, and Receptive Language; PDDBI Express, PDDBI Expressive Language; PDDBI EXSCA/C, PDDBI Expressive Social Communication Abilities Composite; PDDBI REXSCA/C, PDDBI Receptive/Expressive Social Communication Abilities Composite; PDDBI Ritual, PDDBI Ritualisms/Resistance to Change; ABC, Aberrant Behavior Checklist; PDDBI SEMPP, PDDBI Semantic/Pragmatic Problems; PDDBI AGG, PDDBI Aggressiveness; PDDBI SOCAPP, PDDBI Social Approach Behaviors.
Correlations are likely artificially high because of the small sample size.
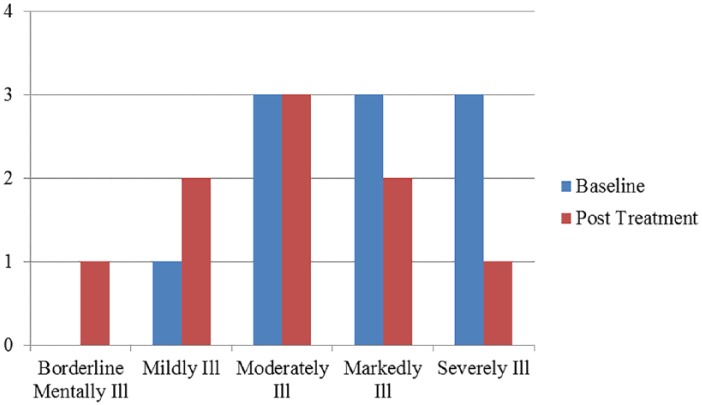
On the CGIS, 6 boys received improved Illness Severity ratings from baseline to post treatment. Most often, ratings shifted to the next, less severe category; however, for 1 child, ratings shifted from severely to moderately affected (see Figure 1). Efficacy ratings per the CGIS reflected a combination of perceived improvement relative to side effects, the latter of which was largely based on parent anecdotal report and the family’s adherence to study regimens. Five children were rated as showing improvement, and 2 children were rated as experiencing significant side effects (one of whom was rated as exhibiting decided improvement with partial remission of symptoms). Efficacy ratings for each child are provided in Table 1, along with a description of what each score means.
Figure 1.
Examiner ratings on the Clinical Global Impression Scale at baseline and post treatment.
Discussion
To our knowledge, this is the first study to examine the effects of high-dose carnitine supplementation on levels of free plasma carnitine and ASD symptoms/behaviors, as well as possible associations between the two, among a sample of young males with ASD. A prior trial of low-dose levocarnitine in children with ASD noted improvement in symptoms via the CARS, a modified global impression score (clinician rated), and the Speech and Cognitive subscales of the ATEC.13 The current study differed in its design (open-label, nonrandomized, pilot trial), higher carnitine dosage (200 mg/kg/day increasing to 400 mg/kg/day), and use of multiple behavioral measures (ADOS, SCQ, PDDBI, AIM, ABC, CGIS). Additional advantages of the current work were that (1) participating children had been rigorously phenotyped at baseline and (b) use of the ADOS at post treatment afforded a standardized context for clinicians’ ratings on the CGIS. In both studies, carnitine levels increased significantly with supplementation, as expected.
With regard to dose compliance and side effects/adverse events, most children tolerated the initial low dose of carnitine (200 mg/kg/day) with minimal side effects, most often fishy odor and/or diarrhea. Only one child reported no side effects. Up-titration to 400 mg/kg/day sometimes increased the severity of side effects, and 3 participants returned to the initial dose. Attrition was likely affected by a combination of perceived behavioral benefit and severity of side effects, as the 2 children who discontinued at mid-treatment reportedly had no noticeable behavioral changes, and one of these experienced fishy odor to the point that the mother was concerned about school bullying. While fishy odor and diarrhea are generally considered “unpleasant,” the cost-benefit ratio is likely balanced differently for each family. For example, another child maintained higher-dose supplementation with reported heavy odor, but the family completed the trial because they observed behavioral improvements.
In terms of behavioral outcomes, it is difficult to compare results between our study and Geier et al’s study13 because different measures were used. However, general similarities are evident in terms of improvements in overall ASD symptoms (via the CARS and SCQ) and some language ratings (via the ATEC Speech subscale and the PDDBI Expressive Social Communication Abilities Composite and Receptive/Expressive Social Communication Abilities Composite scores). While Geier et al13 did not detect changes on the Sociability subscale of the ATEC, our study noted improvements in additional socialization domains (ie, PDDBI Social Pragmatic Problems, PDDBI Social Approach Behaviors, AIM Social-Emotional Reciprocity Impact score). Furthermore, parent ratings on the ABC showed improvements in hyperactivity. The broader range of measures included in the current work may have afforded better detection of changes in these areas. Alternately, because our study was open label, parents may have been more likely to endorse improvements across different behaviors.
Favorable outcomes reported by parents were consistent with the generally mild behavioral improvements noted by clinicians per the CGIS. Six of the 9 children with these data were rated by clinicians as demonstrating some degree of improvement, with 2 rated as “much improved.” None were rated as worse at post treatment. It is possible that children were more comfortable/compliant with post-treatment testing because of familiarity with the clinical setting/rater, or that examiner ratings were biased because of the open-label design. However, behavioral outcomes were associated with increased carnitine levels at post treatment, which was also observed in Geier et al’s13 randomized, double-blinded, controlled design. It remains possible that more favorable outcomes would be observed among a younger sample of children where there may be greater developmental plasticity and responsiveness to carnitine treatment.
Limitations and Future Directions
While the current study focused on a well-characterized group of young males with ASD and gathered standardized data from multiple sources, there are important limitations to consider. The sample size was small and may have reduced power to detect more significant effects. Furthermore, the sample was heterogeneous for age, cognitive functioning, language abilities, and ASD severity. Thus, such a small sample precluded examination of multivariate models and interaction terms, which could elucidate factors that mediate/moderate potential effects of carnitine treatment on behavioral outcomes. This pilot trial was also open label, so parents and clinicians may have been biased in reporting behavioral improvements.
Next-step studies could address these limitations by implementing a double-blinded, randomized, placebo-controlled design and enrolling a larger, younger, and more homogeneous sample. However, the challenge is developing a placebo with comparable odor to the carnitine supplement (to retain blinding), which subsequently influences the degree to which families can be retained. Considering that several boys remained on a low/reduced dose (200 mg/kg/day) of carnitine after the initial increase, lower dosing may reduce unfavorable side effects without sacrificing behavioral benefit. Future work may also seek to include measures that capture the positive qualitative caregiver observations in a standardized fashion.
Footnotes
Authors’ Note: Dr Christian Schaaf is now primarily employed at the Institute of Human Genetics at the University Hospital Cologne, Germany. Anna Laakman is now affiliated with the Center for Autism & Neurodevelopmental Disorders at the University of California, Irvine. Lauren Dowell is no longer affiliated with Baylor College of Medicine or Texas Children’s Hospital.
Author Contributions: RPG-K, FS, CPS, and ALB: conceptualized the study.
FS, CPS, and ALB: conducted physical examinations and provided medical oversight for the study.
RPG-K, LNB, KPN, ALL, and LRD: conducted behavioral/developmental assessments.
DD: coordinated the study and collected data.
AL: facilitated recruitment and shared data.
CGM: analyzed the data and co-wrote the results.
RPG-K, FS, CPS, ALB, and DD: wrote the manuscript, with significant input from LNB, KPN, CGM, AL, ALL and LRD in their respective areas of expertise.
All authors reviewed and approved of the final draft.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Robin P. Goin-Kochel is contracted with Yamo Pharmaceuticals, LLC, to consult on clinical-trial research design. Arthur Beaudet is an advisor to the Baylor Genetic Laboratories (BGL), which is a for-profit joint venture partially owned by Baylor College of Medicine and a majority owned by Miraca Holdings of Japan. BGL offers various forms of genetic testing. Fernando Scaglia receives support to conduct clinical trials in mitochondrial disease from Edison, Rator, Reata, and Stealth Pharmaceuticals. The other authors have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by a grant from the Simons Foundation (SFARI SSC-15 to RGK and ALB). We are grateful to all of the families at the participating SSC sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We appreciate obtaining access to phenotypic data on SFARI Base. This research was also partially supported by the Intellectual and Developmental Disabilities Research Center (1U54 HD083092) at Baylor College of Medicine. We are further grateful for the support of the Willam Stamps Farish Fund. Dr Schaaf’s work was generally supported by the Joan and Stanford Alexander Family. Dr Schaaf is a recipient of a 2011 Clinical Scientist Development Award by the Doris Duke Charitable Foundation.
ORCID iDs: Robin P. Goin-Kochel  https://orcid.org/0000-0001-6666-4369
https://orcid.org/0000-0001-6666-4369
Alvin Loh  https://orcid.org/0000-0003-1102-0843
https://orcid.org/0000-0003-1102-0843
References
- 1. Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103-110. [DOI] [PubMed] [Google Scholar]
- 2. Heil KM, Schaaf CP. The genetics of autism spectrum disorders—a guide for clinicians. Curr Psychiatry Rep. 2013;15:334. [DOI] [PubMed] [Google Scholar]
- 3. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond). 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scaglia F, Longo N. Primary and secondary alterations of neonatal carnitine metabolism. Semin Perinatol. 1999;23:152-161. [DOI] [PubMed] [Google Scholar]
- 5. Ellaway C, Williams K, Leonard H, Higgins G, Wilcken B, Christodoulou J. Rett syndrome: randomized controlled trial of L-carnitine. J Child Neurol. 1999;14:162-167. [DOI] [PubMed] [Google Scholar]
- 6. Longo N, di San Filippo CA, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142C:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celestino-Soper PB, Shaw CA, Sanders SJ, et al. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum Mol Genet. 2011;20:4360-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Celestino-Soper PBS, Violante S, Crawford EL, et al. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Natl Acad Sci U S A. 2012;109:7974-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nava C, Lamari F, Héron D, et al. Analysis of the chromosome X exome in patients with autism spectrum disorders identified novel candidate genes including TMLHE. Transl Psychiatry. 2012;2:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beaudet AL. Brain carnitine deficiency causes nonsyndromic autism with an extreme male bias: a hypothesis. Bioessays. 2017;39(8). doi: 10.1002/bies.201700012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1994. [Google Scholar]
- 12. Rimland B, Edelson SM. Autism Treatment Evaluation Checklist (ATEC). San Diego, CA: Autism Research Institute; 1999. https://www.autismangelspurse.com/wp-content/uploads/2016/10/AutismTreatmentEvaluationChecklist.pdf. Accessed January 30, 2019. [Google Scholar]
- 13. Geier DA, Kern JK, Davis G, et al. A prospective double-blind, randomized clinical trial of levocarnitine to treat autism spectrum disorders. Med Sci Monit. 2011;17:PI15-PI23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192-195. [DOI] [PubMed] [Google Scholar]
- 15. Mullen E. The Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 16. Elliott CD. Differential Ability Scales. 2nd ed. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 17. Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview™, Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 18. Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule—WPS (ADOS-WPS). Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 19. Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule: ADOS-2. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- 20. Rutter M, Bailey A, Lord C. Manual for the Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 21. Cohen IL, Sudhalter V. The PDD Behavior Inventory. Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- 22. Kanne SM, Mazurek MO, Sikora D, et al. The Autism Impact Measure (AIM): initial development of a new tool for treatment outcome measurement. J Autism Dev Disord. 2014;44:168-179. [DOI] [PubMed] [Google Scholar]
- 23. Aman MG, Singh NN. Aberrant Behavior Checklist Manual. East Aurora, NY: Slosson Educational Publications; 1986. [Google Scholar]
- 24. Guy W. ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publication No. ADM 76-338). Rockville, MD: US Department of Health, Education, and Welfare, Public Health Services, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:218-222. [Google Scholar]