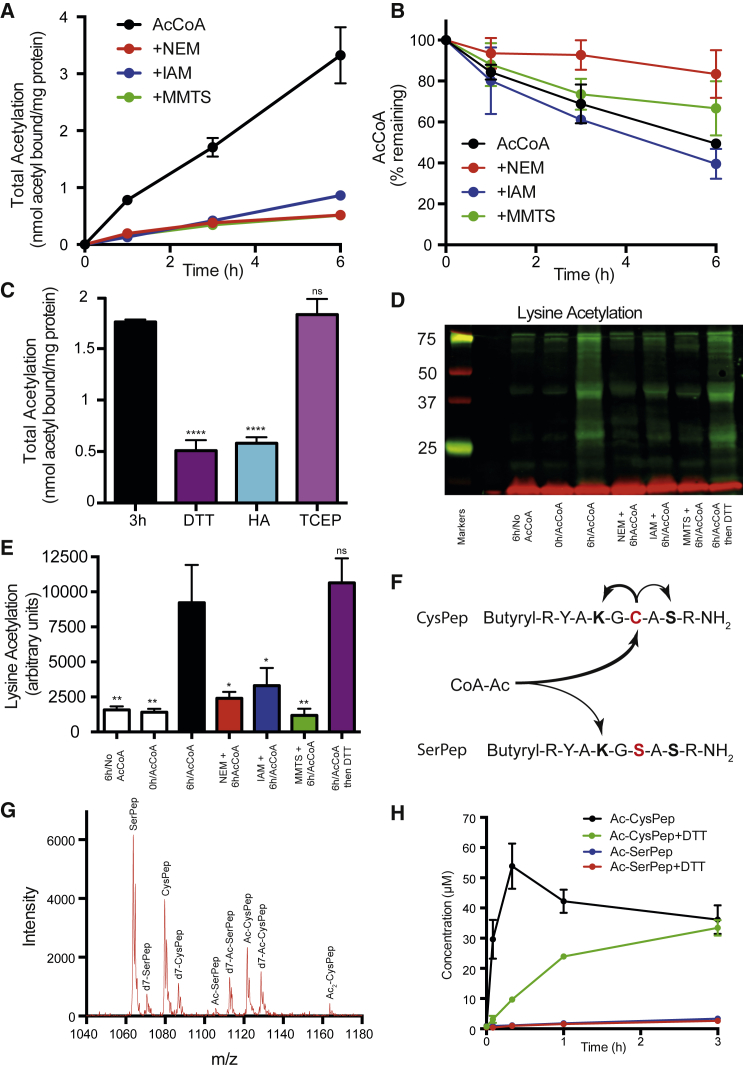
Figure 2.
AcCoA Acetylates Lysine Residues via a Proximal Acetylcysteine Intermediate
(A) Total acetylation is sensitive to thiol-blocking reagents. Mitochondrial membranes were incubated with 2 mM 14C-AcCoA and 25 mM NEM, IAM, or MMTS at 37°C for up to 6 hr.
(B) Alkylating reagents do not break down AcCoA. Mitochondrial membranes were incubated with 2 mM AcCoA and 25 mM NEM, IAM, or MMTS at 37°C for up to 6 hr. AcCoA was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (n = 2 ± range).
(C) Total acetylation is sensitive to thioester-cleaving reagents. Mitochondrial membranes were incubated with 2 mM 14C-AcCoA at 37°C for 3 hr. Afterward, 20 mM DTT, HA, or TCEP was added for 30 min at 37°C.
(D and E) N-acetylation is sensitive to thiol-blocking reagents. (D) Mitochondrial membranes were incubated with 2 mM AcCoA and 25 mM NEM, IAM, or MMTS at 37°C for 6 hr. For others, 20 mM DTT was added for 30 min after the reaction. After reducing SDS-PAGE, acetyllysine (green) and NDUFB8 (red) were visualized. (E) The extent of lysine acetylation was quantified (n = 3 ± SD).
(F–H) Proximal thiol facilitates lysine acetylation as is shown in (F). A cysteine-containing peptide (CysPep; 200 μM) and a serine-containing peptide (SerPep; 200 μM) were coincubated with 200 μM AcCoA. To differentiate S-acetylcysteine from N-acetyllysine and O-acetylserine, 20 mM DTT was added for 30 min after the reaction. A mix of deuterated (d7) standards was then added to quantify acetylation (Ac-SerPep and Ac-CysPep) by MALDI-TOF. The spectrum in (G) is after 3 hr with DTT added. (H) The extent of acetylation was quantified (n = 3 ± SD).
All data are the mean ± SEM of at least three experiments unless otherwise stated. NS, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001. See also Figures S1 and S2.