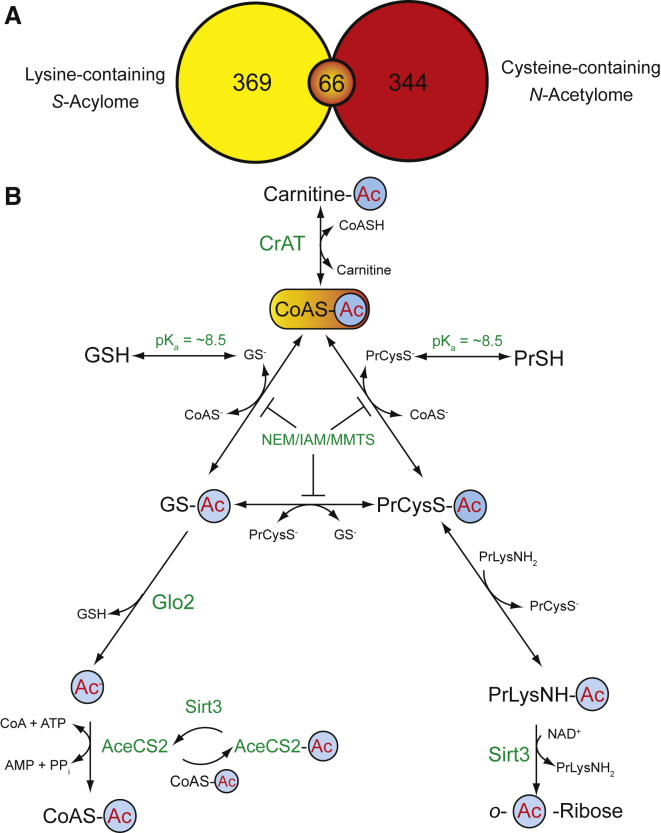
Figure 4.
Model of Thiol Involvement in Non-enzymatic Protein Acetylation
(A) Coexistence of N-acetyllysine and S-acylcysteine. Overlap (orange) between lysine-containing peptides in the mouse liver S-acylome (yellow) (Gould et al., 2015) and cysteine-containing peptides (red) in the mouse liver mitochondrial N-acetylome (blue) (Rardin et al., 2013).
(B) Model of non-enzymatic acetylation. The steady-state concentration of reactive AcCoA is buffered lower by carnitine and carnitine acetyltransferase (CrAT). The pKa and relative concentrations of GSH and protein thiols (PrCysSH) determine the rate at which AcCoA reacts with their thiolate forms, GS− and PrCysS−. NEM, IAM, and MMTS limit reversible thioester exchange by blocking thiols. Glo2 shifts the equilibrium away from cysteine-bound acetyl groups (PrCysSAc) by hydrolyzing S-acetylglutathione to acetate (Ac−). PrCysSAc not removed by GSH can irreversibly transfer to a nearby lysine (PrLysNH2), where Sirt3 can degrade it. AcCoA is regenerated by AcCoA synthetase (AceCS2), and this is regulated by N-acetylation and Sirt3.