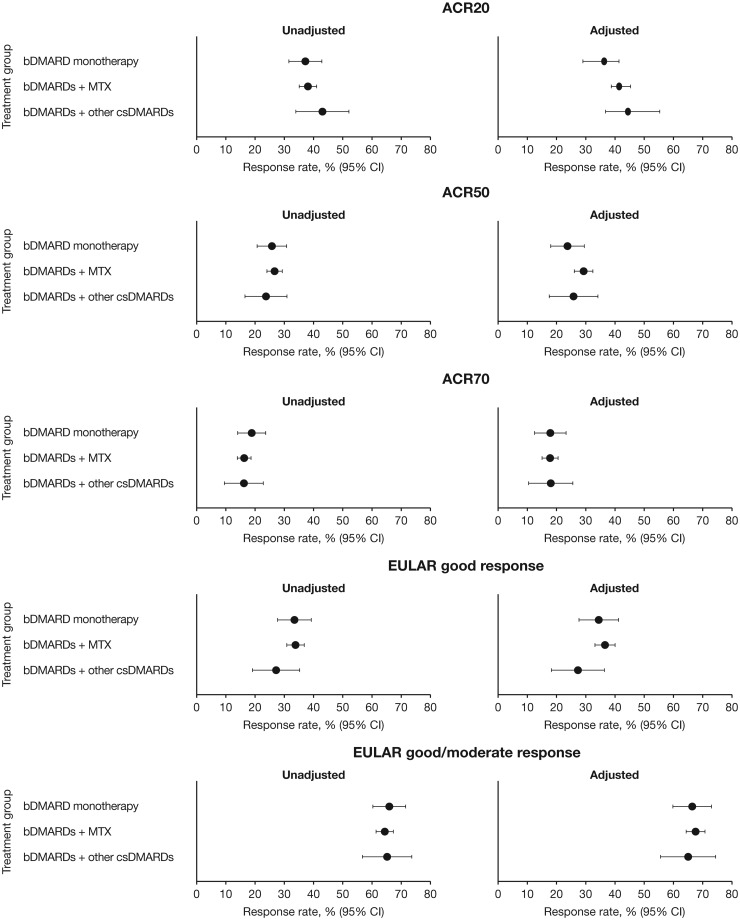
Fig. 2.
Estimated rates of ACR20/50/70 and EULAR good and good/moderate responses at 6 months
bDMARD monotherapy: n = 396; bDMARDs + MTX: n = 1460; bDMARDs + other csDMARDs: n = 208. Adjusted means have been adjusted for sex, age, baseline DAS28-4(ESR) score, time from diagnosis to baseline and use of previous bDMARD treatment. ACR20/50/70: proportion of patients achieving ≥20%, ≥50% or ≥70% improvement, respectively, in the number of tender and swollen joints and ≥3 of 5 ACR components. EULAR good response: proportion of patients achieving improvements from baseline in DAS28-4(ESR)>1.2 in patients with DAS28-4(ESR)≤3.2 at current visit. EULAR moderate response: proportion of patients achieving improvements from baseline in DAS28-4(ESR) >0.6 to ≤1.2 in patients with DAS28-4[ESR]≤3.2 or >3.2 to ≤5.1 at current visit, or improvements from baseline >1.2 in patients with DAS28-4(ESR) >3.2 to ≤5.1 or DAS28-4(ESR)<5.1 at current visit. bDMARD: biologic DMARD; csDMARDs: conventional synthetic DMARDs.