Abstract
We report an extremely rare case of a hybrid tumour of the maxillary sinus. A 51-year-old man presented with a 6-week history of nasal congestion and epiphora. Radiological imaging demonstrated a maxillary sinus tumour, with extensive local invasion. Surgical excision included maxillectomy, left eye exenteration and free flap closure. Histology of the excised specimen showed a rare hybrid tumour containing adenoid cystic carcinoma, salivary duct carcinoma, epithelial-myoepithelial carcinoma and basal cell adenoma. Hybrid tumours are very rare tumour entities which are composed of at least two distinct tumour types. Each tumour entity conforms with a defined tumour type. The tumour entities of a hybrid tumour are not separated but have an identical origin within a definite topographical area. Diagnosis and appropriate management requires high index of suspicion, pathological endeavour to look for a more aggressive accompanying tumour and adequate oncological treatment according to the highest grade of tumour.
Keywords: ear, nose and throat/otolaryngology; pathology; head and neck cancer
Background
Never before has a case been reported in the literature of a maxillary sinus tumour with four primary tumour types. This combination poses unique diagnostic as well as therapeutic challenges.
Case presentation
A 51-year-old man presented to our hospital with a 6-week history of left unilateral nasal congestion, bloody rhinorrhea and epiphora. He had a 15-pack-year smoking history but no other significant medical history. He had no contributory family history. Examination revealed proptosis, opthalmoplegia and a large mass filling the left nasal cavity on nasendoscopy.
Investigations
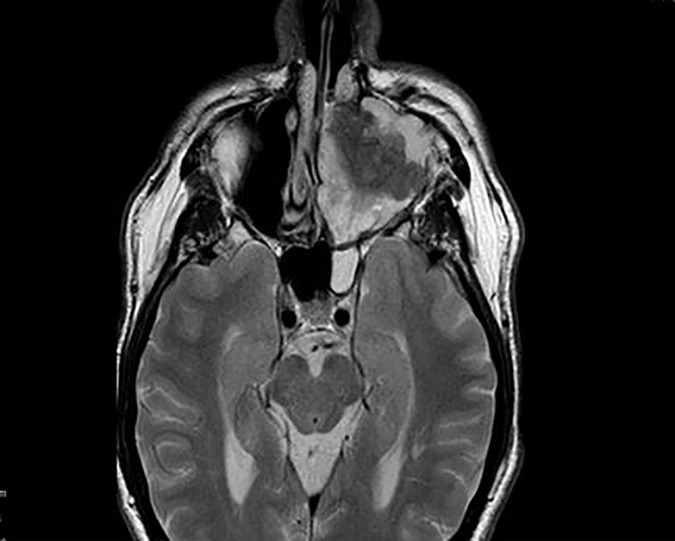
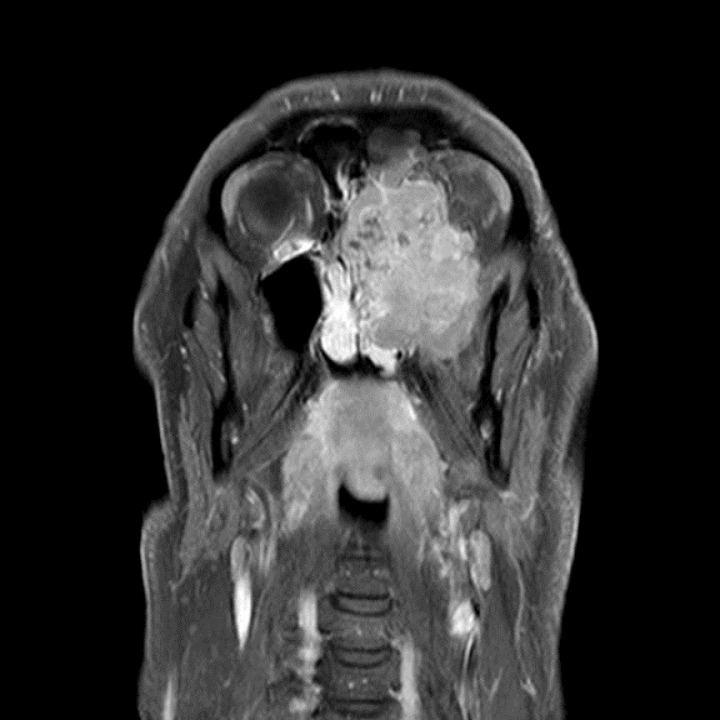
On MRI a left maxillary sinus tumour (figures 1 and 2) was found extending superiorly to the cribiform plate and into the left frontal sinus. There was also considerable intraorbital invasion including breach of the periorbital fascia. PET CT demonstrated an FDG avid tumour with no regional or distant metastases. Biopsies taken preoperatively suggested carcinoma of the maxillary sinus.
Figure 1.
MRI demonstrating maxillary sinus tumour.
Figure 2.
MRI demonstrating coronal view of maxillary sinus invasion.
Treatment
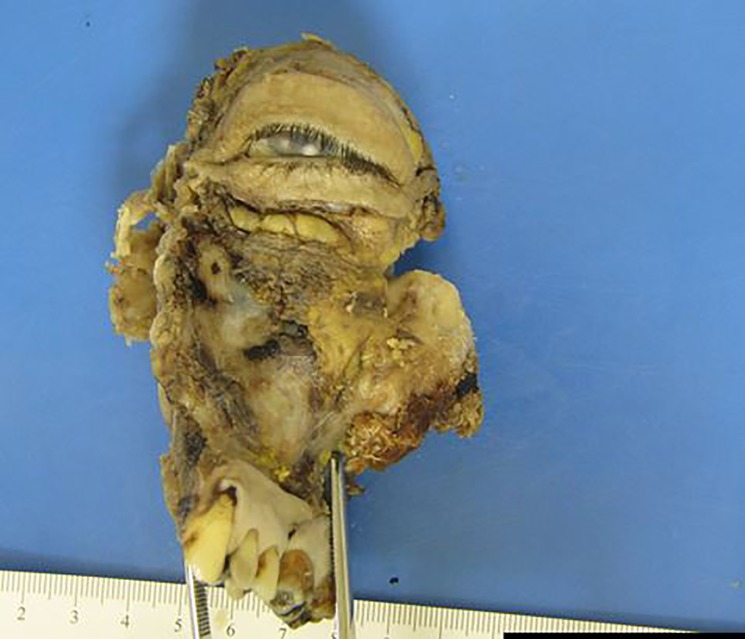
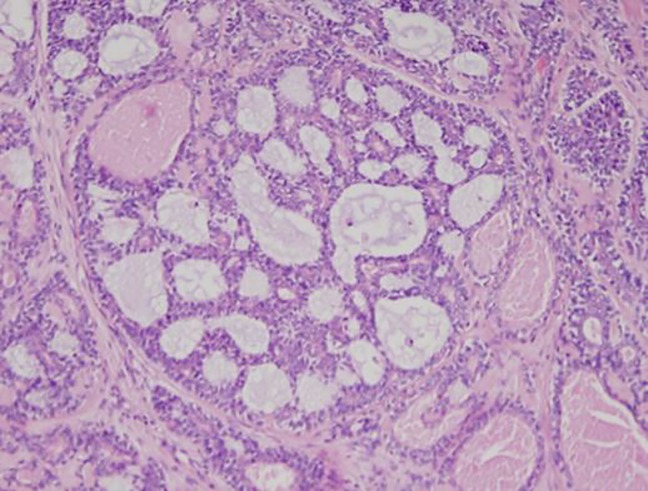
A management plan was constructed at the head and neck oncological multidisciplinary team meeting involving surgical resection and adjuvant treatment following pathological assessment. The surgical specimen included an extended maxillectomy to the cribiform plate and left globe exenteration. An anteriolateral thigh free flap was used to repair the defect in the oral cavity and the facial skin was closed primarily. Specimens were sent for histological examination, including that of the left maxillectomy, left orbital ethmoid and left palate resection measuring 110×72×50 mm (figure 3). The tumour occupied the left maxillary sinus, breaking through the lateral wall of the nose at the level of the middle turbinate, infiltrating the periorbital fascia. It was composed of gland-like and duct-like structures displaying a number of different patterns, including adenoid cystic carcinoma (figure 4) and salivary duct ca. There was prominent background fibrosis and the tumour invaded the bone. The result of immunostaining for the assessment of HER 2 protein (Ventana 4B%) overexpression in the tumour was negative. ER and PR staining was found to be negative.
Figure 3.
Excised histological specimen.
Figure 4.
Adenoid cystic carcinoma component.
Outcome and follow-up
A hybrid tumour containing four primary cell types: adenoid cystic carcinoma (AdCC) containing a solid component, salivary duct carcinoma, basal cell adenoma and epimyoepithelial carcinoma. The patient was treated in accordance to the highest grade of tumour (adenoid cystic or high grade salivary duct carcinoma) and therefore he was commenced on a course of adjuvant radiotherapy. Novel targeted therapy was not added as none were deemed suitable for this patient nor his tumour type. He experienced excellent local control, however he developed a single spinal met in T11 vertebral body 3 years following which was found to be adenoid cystic in nature following biopsy and which was managed with spinal fusion. He is currently doing well and continues to be closely monitored from this point of view at 4 years postoperative involving 6–12 months interval CT scans as well as spinal plain films as well as CT.
Discussion
Sinonasal malignancies (SNMs) often present at an advanced stage. They account for 3% of upper aerodigestive tract tumours and the majority of those are squamous cell carcinomas. Malignant tumours of the minor salivary glands in turn account for 4%–8% of SNM.1 The evolution of cellular pathology techniques mainly relating to immunohistochemistry and fluorescence in situ hybridisation as part of the diagnostic work up of these patients has culminated in the evolution of their classification and the documentation of several new entities.2
Hybrid tumours are very rare tumour entities which are composed of at least two distinct tumour types.3 Each tumour entity conforms with a defined tumour type. The tumour entities of a hybrid tumour are not separated but have an identical origin within a definite topographical area.4 These tumours should not be confused with certain entities of the salivary glands that may exhibit two or more different morphologies in a single lesion, such as collision tumours (two malignant tumours abut each other but actually arise from separate primary sites), tumours with biphasic differentiation (single entities containing two different cellular types, such as pleomorphic adenomas), malignancies arising from a pre-existing benign tumour, as in carcinomas ex mixed tumour, synchronous and multiple tumours, carcinomas with metaplastic change, sarcomatoid salivary duct carcinoma and adenosquamous carcinoma.5–8
In the tissue samples of more than 6600 salivary gland tumours covered by the Salivary Gland Register (Institute of Pathology, University of Hamburg, Germany) only five cases of hybrid tumours, just two of which were malignant, were recorded between 1965 and 1994.3 Their incidence has been estimated between <0.1%–0.4% of all salivary gland tumours.3 5 Comprehensive analysis of case reports and case series since the term hybrid tumour was first introduced by Siefert and Donath in 19963 reveals 33 case in the literature, (18 men, 15 women), with median age of 61.5 years and range of 26–81 years. Two were composed of four tumours, with the rest having two cell types, giving a total of 70 distinct tumours. AdCC (20), salivary duct carcinomas (SDC) (15) and epithelial-myoepithelial carcinoma (EMC) (15) are the three most common tumour types, with a total of 12 different tumour types reported. The combinations of SDC/AdCC and EMC/AdCC were the most common hybrid tumours both occurring on seven occasions. The majority (66.6%) occurred in the major salivary glands (parotid-18, submandibular gland-3, and sublingual gland-1) and the remaining 33.3% were in the minor salivary glands.
Salivary gland carcinoma has recently gained recognition as its own distinct entity. Therefore the incidence is markedly higher that originally thought, making up 12% of all primary salivary gland tumours.9 Luk et al 9 remains one of the most comprehensive studies evaluating 190 primary salivary gland cancers over a period of 25 years, identifying 23 cases of Savlivary duct carcinoma. Despite 96% of the patients receiving adjuvant therapy, the 5-year disease-free survival was still found to be 36%.9 The dismal prognosis underscores the need for systemic therapy targeting androgen receptor positivity such as HRAS, AKT1, PIK3CA and NRAS. Adenoid cystic tumours pose a unique challenge due to its aggressive behaviour and the difficulty involved in obtaining clear margins surgically.10 At given years, almost 50% of patients develop distant mets and local recurrences may develop even later, therefore long term follow-up is required.10 Grade III Adenoid cycstic carcinoma (solid type) are associated with poorer outcome and have tendency for early development of distant metastasis.11
The biological behaviour and the prognosis of a hybrid tumour are determined by the most aggressive of the coexistent carcinomas. Therefore, treatment in each case should be performed according to the more aggressive component, which is the therapeutic recommendation in all cases of hybrid tumours of salivary gland origin.6 7 12 13
Learning points.
Hybrid tumours are very rare tumour entities which are composed of at least two different tumour types.
They must be treated as per the most aggressive subtype.
A limited experience of hybrid tumours reported makes prognostic assessment and clinical management of these lesions particularly challenging.
Footnotes
Patient consent for publication: Obtained.
Contributors: AW-G: literature review, case compilation and review. PL: literature review, patient follow-up and editing. EO’R: pathological assessment, interpretation and literature review. CT: surgeon and primary care-giver. Editing and review of paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Pantvaidya GH, Vaidya AD, Metgudmath R, et al. . Minor salivary gland tumors of the sinonasal region: results of a retrospective analysis with review of literature. Head Neck 2012;34:1704–10. 10.1002/hed.21988 [DOI] [PubMed] [Google Scholar]
- 2. Badlani J, Gupta R, Balasubramanian D, et al. . Primary salivary gland malignancies: a review of clinicopathological evolution, molecular mechanisms and management. ANZ J Surg 2018;88 10.1111/ans.14201 [DOI] [PubMed] [Google Scholar]
- 3. Seifert G, Donath K. Hybrid tumours of salivary glands. Definition and classification of five rare cases. Eur J Cancer B Oral Oncol 1996;32B:251–9. 10.1016/0964-1955(95)00059-3 [DOI] [PubMed] [Google Scholar]
- 4. Kamio N, Tanaka Y, Mukai M, et al. . A hybrid carcinoma: adenoid cystic carcinoma and salivary duct carcinoma of the salivary gland. An immunohistochemical study. Virchows Arch 1997;430:495–500. 10.1007/s004280050060 [DOI] [PubMed] [Google Scholar]
- 5. Nagao T, Sugano I, Ishida Y, et al. . Hybrid carcinomas of the salivary glands: report of nine cases with a clinicopathologic, immunohistochemical, and p53 gene alteration analysis. Mod Pathol 2002;15:724–33. 10.1097/01.MP.0000018977.18942.FD [DOI] [PubMed] [Google Scholar]
- 6. Ruíz-Godoy LM, Mosqueda-Taylor A, Suárez-Roa L, et al. . Hybrid tumours of the salivary glands. A report of two cases involving the palate and a review of the literature. Eur Arch Otorhinolaryngol 2003;260:312–5. 10.1007/s00405-002-0566-7 [DOI] [PubMed] [Google Scholar]
- 7. Woo JS, Kwon SY, Jung KY, et al. . A hybrid carcinoma of epithelial-myoepithelial carcinoma and adenoid cystic carcinoma in maxillary sinus. J Korean Med Sci 2004;19:462–5. 10.3346/jkms.2004.19.3.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kainuma K, Oshima A, Suzuki H, et al. . Hybrid carcinoma of the parotid gland: report of a case (epithelial-myoepithelial carcinoma and salivary duct carcinoma) and review of the literature. Acta Otolaryngol 2010;130:185–9. 10.3109/00016480902930458 [DOI] [PubMed] [Google Scholar]
- 9. Luk PP, Weston JD, Yu B, et al. . Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck 2016;38(Suppl. 1):E1838–47. 10.1002/hed.24332 [DOI] [PubMed] [Google Scholar]
- 10. van Weert S, Bloemena E, van der Waal I, et al. . Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol 2013;49:824–9. 10.1016/j.oraloncology.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 11. Stallmach I, Zenklusen P, Komminoth P, et al. . Loss of heterozygosity at chromosome 6q23–25 correlates with clinical and histologic parameters in salivary gland adenoid cystic carcinoma. Virchows Arch 2002;440:77–84. 10.1007/s004280100523 [DOI] [PubMed] [Google Scholar]
- 12. Croitoru CM, Suarez PA, Luna MA. Hybrid carcinomas of salivary glands. Report of 4 cases and review of the literature. Arch Pathol Lab Med 1999;123:698–702. [DOI] [PubMed] [Google Scholar]
- 13. Zardawi IM. Hybrid carcinoma of the salivary gland. Histopathology 2000;37:283–4. 10.1046/j.1365-2559.2000.01020-2.x [DOI] [PubMed] [Google Scholar]