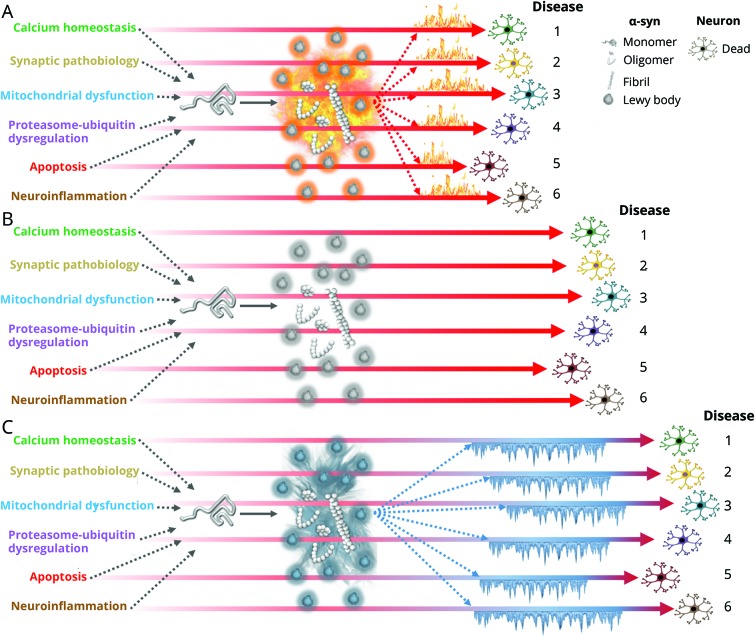
Figure 3. Alternative models of protein aggregation in Parkinson disease (multiple disease model).
Abnormal soluble oligomers and fibrils of α-synuclein (α-syn), while not directly pathogenic, act as accelerators of neurodegeneration (“fueling the fire”) due to early pathogenic molecular abnormalities, each representing molecularly distinct diseases (A, model 1). Alternatively, abnormal soluble oligomers and fibrils of α-synuclein aggregate into Lewy bodies as byproducts of earlier pathogenic molecular mechanisms, without directly affecting the neurodegenerative process brought on by each molecular disease (B, model 2). Finally, α-synuclein aggregates into Lewy bodies as a mechanism to protect the neuron from toxic protein species or from the biological dysfunction that may have generated the formation of toxic species, “cooling” progression of cell degeneration under biological stress (C, model 3). Note that each molecularly defined disease has a different time to death; collectively, time to neuronal death is longest in diseases corresponding to model C.