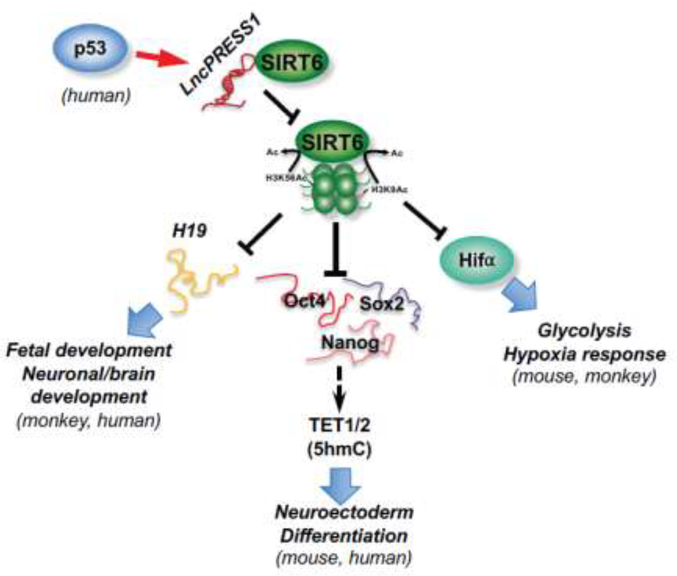
Figure 2. SIRT6 epigenetically regulates proper lineage commitment of ESCs and animal development.
In mouse, monkey, and human, SIRT6 represses expression of metabolic genes (particularly glycolytic genes), core pluripotent genes, and maternal imprinted lncRNA H19 through deacetylation of H3K9ac and K3K56ac. These actions of SIRT6 metabolically and epigenetically safeguard the balance between pluripotency and differentiation. In hESCs, a p53-targeted lncRNA, lncPRESS1, physically interacts with SIRT6 and prevents its chromatin localization, which in turn maintains high levels of H3K56ac and H3K9ac at promoters of pluripotency genes. Therefore, in contrast to SIRT1, SIRT6 is an evolutionally conserved regulator of ESC differentiation and animal development both in vitro and in vivo.