Key Points
Question
What are the prevalence of antidementia treatment among older adults with Parkinson disease and the sociodemographic and geographic factors associated with inappropriate prescribing for these individuals?
Findings
In this cross-sectional study of data from 268 407 Medicare beneficiaries with Parkinson disease, of those who received antidementia treatment, nearly 45% experienced the never event of coadministration of a high-potency anticholinergic medication and an acetylcholinesterase inhibitor. Women and Hispanic beneficiaries as well as those living in the southern and midwestern US states were at highest odds of inappropriate dementia drug prescribing.
Meaning
These findings may be useful toward the development of targeted interventions to minimize geographic and demographic disparities in dementia care.
Abstract
Importance
Dementia is common in Parkinson disease, but few data exist on dementia treatment patterns or the concurrent use of acetylcholinesterase inhibitors (ACHEIs) and anticholinergic medications, a frank prescribing error.
Objectives
To describe dementia treatment patterns, and to determine the extent to which the concurrent use of ACHEIs and drugs with strong anticholinergic activity occurs among individuals with Parkinson disease in the United States.
Design, Setting, and Participants
This cross-sectional analysis included adult Medicare beneficiaries (aged 65 years or older) with Parkinson disease diagnosis with 12 consecutive months of inpatient, outpatient, and prescription drug coverage from January 1, 2014, through December 31, 2014. Beneficiaries with other parkinsonian syndromes were excluded. Demographic, geographic, prescription claims, and other data were extracted from the 2014 Carrier, Beneficiary Summary, and Prescription Drug Event research identifiable files of the Centers for Medicare & Medicaid Services. Data analysis was conducted from August 1, 2017, to November 30, 2017.
Main Outcomes and Measures
Primary outcomes were use of dementia drug, specific dementia medication, and concurrent exposure to a high-potency anticholinergic drug and an ACHEI. Descriptive analyses and multivariable logistic regression models determined the extent to which patient characteristics and comorbid conditions were associated with dementia treatment or with a high-potency anticholinergic and ACHEI never event.
Results
Of 268 407 Medicare beneficiaries with Parkinson disease (mean [SD] age, 78.9 [7.5]; 134 575 male [50.1%]), most were identified in the files as white (232 831 [86.7%]), followed by black (14 629 [5.5%]), Hispanic (7176 [2.7%]), Asian (7115 [2.7%]), and Native American (874 [0.3%]). Among these beneficiaries, 73 093 (27.2%) were given a prescription for at least 1 antidementia medication. The most commonly prescribed medication was donepezil hydrochloride (46 027 [63.0%] users), followed by memantine hydrochloride (30 578 [41.8%] users) and rivastigmine tartrate (19 278 [26.4%] users). Dementia drugs were more likely to be prescribed to black (adjusted odds ratio [AOR], 1.33; 95% CI, 1.28-1.38) and Hispanic (AOR, 1.28; 95% CI, 1.22-1.35) beneficiaries and less likely for Native American beneficiaries (AOR, 0.62; 95% CI, 0.51-0.74). Women were less likely than men to be given a prescription for dementia medication (AOR, 0.85; 95% CI, 0.84-0.87). Of the 64 017 beneficiaries receiving an ACHEI, 28 495 (44.5%) experienced at least 1 high-potency anticholinergic–ACHEI event. Hispanic (AOR, 1.11; 95% CI, 1.00-1.23) and women (AOR, 1.30; 95% CI, 1.25-1.35) beneficiaries had greater odds of experiencing this never event. Statistically significant clusters of the prevalence of this prescribing error were observed across the United States (Moran I = 0.24; P < .001), with clusters of high prevalence in the southern and midwestern states.
Conclusions and Relevance
Dementia medication use by persons with Parkinson disease varies by race/ethnicity and sex; potentially inappropriate prescribing is common among those being treated for cognitive impairment and varies by race/ethnicity, sex, and geography. These findings may serve as national and local targets for improving care quality and outcomes for persons with Parkinson disease.
This cross-sectional study analyzes data from the Centers for Medicare & Medicaid Services to identify both effective and ineffective dementia medication prescribing patterns as well as patient characteristics and comorbid conditions associated with dementia treatment among older Medicare beneficiaries with Parkinson disease.
Introduction
Among persons with Parkinson disease, cognitive impairment is a major driver of loss of independence, nursing home placement, and health care costs.1,2,3 One in 4 patients with Parkinson disease has objective cognitive impairment at the time of diagnosis,4 and the prevalence of dementia in Parkinson disease reaches 80% to 90% by 12 years after diagnosis.5 The literature on the incidence of cognitive impairment in Parkinson disease is extensive, but to date no national data are available on dementia medication use among US patients with Parkinson disease, and little data exist on use of a dementia drug among women or racial/ethnic minorities with Parkinson disease.
Large-scale data on dementia medication use are necessary to define the clinical scope of the problem of dementia in Parkinson disease, but identifying patterns of ineffective dementia medication use or reversible contributors to cognitive dysfunction has the potential to reduce symptom burden and alter the clinical trajectory of Parkinson disease. Acetylcholinesterase inhibitors (ACHEIs) improve cognition by increasing cholinergic activity and include the most widely used dementia drug in the world, donepezil hydrochloride,6 as well as rivastigmine tartrate7,8 and galantamine hydrobromide.9,10 Drugs that block cholinergic transmission or have anticholinergic activity are prescribed by all clinical specialties; these drugs include some of the most commonly used in the United States, such as oxybutynin chloride, paroxetine hydrochloride, and diphenhydramine hydrochloride. In the general adult population, anticholinergic medication use is associated with worse performance on cognitive testing,11,12 increased risk of dementia,13,14,15,16 falls,17 diminished health-related quality of life,18 and higher health service utilization.19 Patients with Parkinson disease may be even more vulnerable to the adverse effects of anticholinergic drugs because of the disease-related disruption of central cholinergic pathways.20
Coadministration of a drug with high anticholinergic activity and an ACHEI represents a frank prescribing error because these drugs have opposing pharmacologic effects. In patients with Parkinson disease, who bear additional risks of cognitive impairment and vulnerability to anticholinergic activity, coprescribing of an ACHEI and a high-potency anticholinergic medication can be considered a never event because it is a medication error likely to contribute to disability. To our knowledge, no data are available on the occurrence of this prescribing error in the Parkinson disease population. To address these gaps in knowledge, we established these study objectives to: (1) describe dementia treatment patterns and (2) determine the extent to which concurrent use of ACHEIs and drugs with strong anticholinergic activity occurs among individuals with Parkinson disease diagnosis in the United States. To provide initial data for community-based or local policy initiatives aimed at reducing avoidable outcomes, we examined the geographic variation in potentially inappropriate prescribing to patients with Parkinson disease.
Methods
This study was approved by the University of Pennsylvania Human Research Protections Office and the Centers for Medicare & Medicaid Services (CMS). These CMS data are made available to researchers with a waiver of consent with an approved Data Use Agreement. We conducted data analysis from August 1, 2017, to November 30, 2017.
Data Set
The Medicare program is the primary insurer for 97% of the US population aged 65 years or older, providing inpatient, outpatient, and prescription coverage.21 This study used data from the 2014 Carrier, Beneficiary Summary, and Prescription Drug Event research identifiable files (RIFs) of the CMS. The RIFs contain individual-level data that can be linked across data sets over time using a beneficiary identification code. The Carrier RIFs contain diagnoses and procedures from physician encounters documented using International Classification of Diseases, Ninth Revision (ICD-9) and Current Procedural Terminology codes. The Beneficiary Summary File contains Medicare enrollment and eligibility information, demographic variables, postal codes, and indicator variables for 27 common chronic medical conditions, which are obtained using validated algorithms.21 The Prescription Drug Event RIFs contain outpatient prescription drug claims for Medicare beneficiaries with Medicare prescription drug coverage; these RIFs include drug name, strength, dose, quantity, dispense date, and days’ supply.
Inclusion and Exclusion Criteria
We included Medicare beneficiaries who had 12 consecutive months of inpatient, outpatient, and prescription drug coverage from January 1, 2014, through December 31, 2014. We identified individuals with Parkinson disease as those with at least 2 outpatient encounter diagnoses for Parkinson disease (ICD-9 code 332.0). We excluded individuals with diagnostic codes for other parkinsonian syndromes (eg, ICD-9 code 333.0 [other diseases of the basal ganglia]).
Prescription Drug Identification
Prescription claims were queried for all drugs with a US Food and Drug Administration indication for dementia. Persons with at least 1 prescription claim for donepezil, rivastigmine, galantamine, or memantine hydrochloride were defined as those who took a dementia drug and underwent query of all other medications prescribed in 2014. Individuals who took donepezil, rivastigmine, or galantamine were further categorized as taking an ACHEI.
Anticholinergic Medication Classification
Several methods exist for ranking relative anticholinergic activity; there is no criterion standard. The Anticholinergic Cognitive Burden Scale (score range: 0-3)11 ranks anticholinergic medications according to their effects on cognition. Drugs with possible anticholinergic activity (in vitro assays) were given a score of 1. Drugs with established and clinically relevant cognitive anticholinergic activity were given a score of either 2 or 3, on the basis of the blood-brain barrier permeability of the medication (eTable 1 in the Supplement).22
Individual risk-benefit assessments may justify the use of low-potency or midpotency anticholinergic drugs in persons with Parkinson disease or with Parkinson disease and dementia, particularly in the absence of evidence that use of such agents affects the clinical trajectory of Parkinson disease. Defending the use of a high-potency anticholinergic drug concurrently with an ACHEI is more difficult, considering that all high-potency anticholinergic drugs have low-potency, nonanticholinergic, and nondrug alternatives.23 Thus, our primary analyses focused on high-potency anticholinergic drugs.
Study Outcomes
The primary study outcomes were use of a dementia drug (yes or no), specific dementia medication, and concurrent exposure to a high-potency anticholinergic drug and an ACHEI (also referred to as a never event in this article). Drug claims were queried to identify the drugs of interest, and the fill date and days’ supply variables were used to calculate the potential boundaries of exposure for each drug.
Covariables
We extracted demographic, clinical, and geographic data to use as covariables. Demographic variables were race/ethnicity, age, and sex. The presence of atrial fibrillation, dementia, depression, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, hypertension, hyperlipidemia, ischemic heart disease, and stroke or transient ischemic attack was determined for each individual, using annual chronic disease indicators. The residential postal code was used to assign each beneficiary to a hospital referral region, a geographic unit that denotes local health care markets in which Medicare beneficiaries receive tertiary medical care.24
Statistical Analysis
We calculated the prevalence of the use of a dementia drug in a sample of individuals with Parkinson disease and described this use according to demographic and comorbid disease variables. Multivariable logistic regression models were built to determine which demographic and comorbid variables were associated with the use of a dementia drug or with concurrent use of a high-potency anticholinergic agent and an ACHEI. We produced choropleth maps of the prevalence of these never events in the United States across hospital referral regions, using Stata, version 15 (StataCorp LLC). To test for nonrandom spatial variation in hospital referral region–level rates of concurrent use of a high-potency anticholinergic drug and an ACHEI, we used SpaceStat, version 3.6 (Biomedware). We calculated the Moran I weighted correlation coefficient to detect spatial clustering. A 2-sided P = .05 was considered statistically significant.
Results
Baseline Characteristics
As displayed in Table 1, a total of 268 407 persons met the inclusion criteria. Of this total, 134 575 (50.1%) were male and 133 832 (49.9%) were female, with a mean (SD) age of 78.9 (7.5) years. Most individuals in the sample were identified in the RIFs as white (232 831 [86.7%]), followed distantly by black (14 629 [5.5%]), Hispanic (7176 [2.7%]), Asian (7115 [2.7%]), and Native American (874 [0.3%]). Table 1 reports the proportion of female Medicare beneficiaries with Parkinson disease, not the prevalence of Parkinson disease among female Medicare beneficiaries. A previous study demonstrated that Parkinson disease is more prevalent among men than women in the Medicare population,25 which is consistent with other studies that demonstrated a sex difference in Parkinson disease risk.26 Older women outnumber older men in the United States: 60% of persons aged 65 years or older and 67% of persons aged 85 years or older are female; this status quo affects the absolute number of women with Parkinson disease. Thus, our finding that similar numbers of male- and female-composed Parkinson disease cases is wholly consistent with a disease that affects the older adult population in which the higher-risk group (males) has lower absolute numbers and/or a lower relative survival. The proportion of Parkinson disease cases in our sample increased with age.
Table 1. Baseline Characteristics of 268 407 Medicare Beneficiaries With Parkinson Disease and Prescription Drug Benefits, 2014.
| Characteristic | No. (%) |
|---|---|
| Race/ethnicitya | |
| White | 232 831 (86.7) |
| Black | 14 629 (5.5) |
| Other/unknown | 5782 (2.2) |
| Asian | 7115 (2.7) |
| Hispanic | 7176 (2.7) |
| Native American | 874 (0.3) |
| Sex | |
| Male | 134 575 (50.1) |
| Female | 133 832 (49.9) |
| Age, y | |
| 65-69 | 32 975 (12.3) |
| 70-74 | 51 161 (19.1) |
| 75-79 | 60 020 (22.4) |
| 80-84 | 57 648 (21.5) |
| ≥85 | 66 603 (24.8) |
| Common comorbid disease | |
| Atrial fibrillation | 33 836 (12.6) |
| CKD | 63 623 (23.7) |
| COPD | 38 449 (14.3) |
| Dementia | 111 736 (41.6) |
| Depression | 93 944 (35.0) |
| Diabetes | 79 430 (29.6) |
| Heart failure | 61 013 (22.7) |
| Hip/pelvic fracture | 6620 (2.5) |
| Hyperlipidemia | 133 270 (49.7) |
| Hypertension | 180 291 (67.2) |
| Ischemic heart disease | 102 073 (38.0) |
| Stroke/TIA | 25 810 (9.6) |
| Dementia medication useb | |
| Any | 73 080 (27.2) |
| None | 195 327 (72.8) |
| ACHEI | 64 017 (23.9) |
| NMDA receptor antagonist | 30 578 (11.4) |
Abbreviations: ACHEI, acetylcholinesterase inhibitor; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NMDA, N-methyl-D-aspartate; TIA, transient ischemic attack.
Race/ethnicity data were not coded separately.
An individual may have had a prescription for more than 1 dementia drug over the course of the year; therefore, specific drug categories are not exclusive.
Use of a Dementia Drug in Parkinson Disease
As shown in Table 2, of the 268 407 Medicare beneficiaries with Parkinson disease in the study sample, 73 093 (27.2%) filled at least 1 prescription for dementia medication in 2014. Among these dementia medications, the most commonly prescribed was donepezil (46 027 [63.0%] users), followed by memantine (30 578 [41.8%]) and rivastigmine (19 278 [26.4%]); galantamine use was uncommon (<3%). At least 1 prescription fill was the basis for the study exposure, but multiple prescription fills may suggest a committed management practice. Excluding individuals who had their first ACHEI fill in December 2014 (as refills in 2015 could not be detected), 41 290 (90.2%) donepezil, 26 626 (87.5%) rivastigmine, and 1875 (88.9%) galantamine prescriptions were refilled at least once.
Table 2. Dementia Medication Use Among 73 093 Medicare Beneficiaries With Parkinson Disease, 2014a.
| Variable | No. (%) |
|---|---|
| Dementia drug name | |
| Donepezil hydrochloride | 46 027 (63.0) |
| Galantamine hydrobromide | 2119 (2.9) |
| Rivastigmine tartrate | 19 278 (26.4) |
| Memantine hydrochloride | 30 578 (41.8) |
| Dementia treatment patterns | |
| Donepezil only | 28 815 (39.4) |
| Rivastigmine only | 10 636 (14.6) |
| Memantine only | 9076 (12.4) |
| Donepezil + memantine | 13 987 (19.1) |
| Rivastigmine + memantine | 5549 (7.6) |
| Anticholinergic Cognitive Burden Scaleb | |
| Low potency | 45 861 (62.7) |
| Midpotency | 5517 (7.5) |
| High potency | 30 709 (42.0) |
| Concurrent use of an ACH drug and an ACHEI (n = 64 017 taking an ACHEI) | |
| Any ACH | 52 069 (81.3) |
| Low-potency ACH | 43 554 (68.0) |
| Midpotency ACH | 4892 (7.6) |
| High-potency ACH | 28 495 (44.5) |
Abbreviations: ACH, anticholinergic; ACHEI, acetylcholinesterase inhibitor.
As measured by the presence of at least 1 prescription fill for a given drug or drug category. Categories are not mutually exclusive; a beneficiary could fit into more drug categories over the course of the study period.
The Anticholinergic Cognitive Burden Scale (score range: 0-3)11 ranks anticholinergic medications according to their effects on cognition. Drugs with possible anticholinergic activity (in vitro assays) were given a score of 1. Drugs with established and clinically relevant cognitive anticholinergic activity were given a score of either 2 or 3, on the basis of the blood-brain barrier permeability of the medication (eTable 1 in the Supplement).22
Dementia medication use was positively associated with black (adjusted odds ratio [AOR], 1.33; 95% CI, 1.28-1.38) and Hispanic (AOR, 1.28; 95% CI, 1.22-1.35) race/ethnicity, and it was negatively associated with Native American (AOR, 0.62; 95% CI, 0.51-0.74) and other or unknown (AOR, 0.84; 95% CI, 0.78-0.90) race/ethnicity, as compared with white race/ethnicity. Female sex was associated with lower odds of dementia medication use (AOR, 0.85; 95% CI, 0.84-0.87). Use of a dementia drug was positively associated with age. Medical diagnoses associated with dementia drug prescriptions included dementia (AOR, 7.33; 95% CI, 7.18-7.48), depression (AOR, 1.80; 95% CI, 1.77-1.83), hip or pelvic fracture (AOR, 1.26; 95% CI, 1.20-1.33), and stroke or transient ischemic attack (AOR, 1.29; 95% CI, 1.26-1.33). Chronic obstructive pulmonary disease, diabetes, ischemic heart disease, and hypertension were weakly associated with use of a dementia drug (Table 3).
Table 3. Characteristics and Comorbid Conditions Associated With Dementia Medication Use Among 268 407 Medicare Beneficiaries With Parkinson Disease, 2014.
| Variable | No. (%) | Adjusted Odds Ratio (95% CI)a |
|---|---|---|
| Race/ethnicity | ||
| White | 232 831 (26.8) | 1 [Reference] |
| Black | 14 629 (31.9) | 1.33 (1.28-1.38)b |
| Other/unknown | 5782 (22.4) | 0.84 (0.78-0.90)b |
| Asian | 7115 (27.7) | 0.98 (0.93-1.04) |
| Hispanic | 7176 (35.1) | 1.28 (1.22-1.35)b |
| Native American | 874 (18.3) | 0.62 (0.51-0.74)b |
| Sex | ||
| Male | 134 575 (28.0) | 1 [Reference] |
| Female | 133 832 (26.5) | 0.85 (0.84-0.87)b |
| Age, y | ||
| 65-69 | 32 975 (15.6) | 1 [Reference] |
| 70-74 | 51 161 (21.2) | 1.45 (1.39-1.50)b |
| 75-79 | 60 020 (27.5) | 2.01 (1.94-2.09)b |
| 80-84 | 57 648 (31.9) | 2.46 (2.37-2.55)b |
| ≥85 | 66 603 (33.4) | 2.60 (2.51-2.69)b |
| Common comorbid disease | ||
| Atrial fibrillation | 33 836 (26.9) | 0.88 (0.84-0.91)b |
| CKD | 63 623 (30.1) | 1.11 (1.09-1.13)b |
| COPD | 38 449 (29.3) | 1.10 (1.07-1.13)b |
| Dementia | 111 736 (49.3) | 7.33 (7.18-7.48)b |
| Depression | 93 944 (34.3) | 1.80 (1.77-1.83)b |
| Diabetes | 79 430 (28.3) | 1.06 (1.04-1.08)b |
| Heart failure | 61 013 (29.4) | 1.02 (0.99-1.04) |
| Hip/pelvic fracture | 6620 (33.5) | 1.26 (1.20- 1.33)b |
| Hyperlipidemia | 133 270 (27.0) | 0.99 (0.97-1.00) |
| Hypertension | 180 291 (28.1) | 1.06 (1.04-1.08)b |
| Ischemic heart disease | 102 073 (28.9) | 1.05 (1.03-1.07)b |
| Stroke/TIA | 25 810 (32.9) | 1.29 (1.26-1.33)b |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
As measured using a logistic regression model that included race/ethnicity, age, sex, and (when applicable) indicator variable for the comorbid disease of interest.
Statistically significant estimate.
Advanced Therapy
Memantine is indicated by the US Food and Drug Administration for treatment of moderate and advanced dementia. Memantine use increased with age, which is not surprising, but also varied by race/ethnicity and sex. Of Hispanic beneficiaries being treated for dementia, 1275 (61.1%) were given a prescription for memantine, more than white (25 848 [47.1%]), black (1990 [48.5%]), Asian (837 [49.4%]), and Native American (59 [43.1%]) beneficiaries who received dementia drugs prescriptions. Compared with the white race/ethnicity, Hispanic race/ethnicity was associated with greater adjusted odds of receiving memantine (AOR, 1.42; 95% CI, 1.31-1.53). Female sex was associated with slightly greater odds of memantine use (AOR, 1.08; 95% CI, 1.05-1.11).
Dual Therapy
For almost two-thirds (48 527 [66.4%]) of the study sample, only 1 dementia drug was prescribed over the 12-month observation period, most frequently donepezil (28 815 [39.4%]) followed by rivastigmine (10 636 [14.6%]). Dual dementia drug therapy, indicated by concurrent prescription fills for memantine and an ACHEI, was prescribed to 26.7% of the study sample (19 536), most often donepezil and memantine (13 987 [19.1%]) followed by rivastigmine and memantine (5549 [7.6%]). Compared with white beneficiaries, black beneficiaries had greater adjusted odds of receiving combination therapy with donepezil and memantine (AOR, 1.22; 95% CI, 1.13-1.31). Hispanic beneficiaries had greater odds of receiving both donepezil and memantine (AOR 1.14; 95% CI, 1.03-1.26) and rivastigmine and memantine (AOR 1.38; 95% CI, 1.22-1.57). The galantamine and memantine combination was too infrequently used to generate valid statistical models. The odds of dual therapy increased with age. Sex differences in dual therapy were minimal (eTable 2 in the Supplement).
Concurrent Anticholinergic Drug and ACHEI Use
Of the 64 017 individuals with Parkinson disease being treated for dementia with an ACHEI, 52 069 (81.3%) experienced at least 1 concurrent use of an anticholinergic agent, 43 554 (68%) had a concurrent prescription fill with a low-potency anticholinergic drug, 4892 (7.6%) had a concurrent prescription fill with a midpotency anticholinergic drug, and 28 495 (44.5%) had at least 1 concurrent prescription fill with a high-potency anticholinergic drug (Table 2). Considering the possibility that there was intention to stop the anticholinergic drug therapy (such as verbal or written communication to the beneficiary) that was not reflected in claims data, we determined how frequently sequential high-potency anticholinergic–ACHEI events occurred. Sequential high-potency anticholinergic–ACHEI event was defined as a second concurrent prescription filling, immediately after the first, of a high-potency anticholinergic drug and an ACHEI (donepezil, rivastigmine, or galantamine). We allowed for switching 1 high-potency anticholinergic drug to another, given that the exposure of interest was the concurrent exposure to an ACHEI and a high-potency anticholinergic drug over multiple prescription fill cycles. We found that sequential high-potency anticholinergic–ACHEI events were common: 17 149 persons (84.9%) who filled a prescription for donepezil and a high-potency anticholinergic drug experienced at least 1 event, and 7305 (84.1%) high-potency anticholinergic–rivastigmine events occurred more than once.
As shown in Table 4, regression models that adjusted for demographic characteristics and comorbid diseases found that black beneficiaries were less likely to have concurrent high-potency anticholinergic and ACHEI prescription (AOR, 0.83; 95% CI, 0.77-0.89) compared with whites. Conversely, Hispanic (AOR, 1.11; 95% CI, 1.00-1.23) and women (AOR, 1.30; 95% CI, 1.25-1.35) beneficiaries had greater odds of experiencing this never event. Coadministration of a high-potency anticholinergic drug and an ACHEI was negatively associated with age. It was associated, however, with comorbid depression (AOR, 1.62; 95% CI, 1.57-1.68), chronic obstructive pulmonary disease (AOR, 1.12; 95% CI, 1.03-1.17), hypertension (AOR, 1.13; 95% CI, 1.09-1.19), chronic kidney disease (AOR, 1.08; 95% CI, 1.03-1.12), diabetes (AOR, 1.07; 95% CI, 1.03-1.11), and heart failure (AOR, 1.06; 95% CI, 1.02-1.11). Concurrent use was not associated with comorbid cerebrovascular events, ischemic heart disease, or hyperlipidemia.
Table 4. Adjusted Odds of Concurrent High-Potency Anticholinergic Drug and Acetylcholinesterase Inhibitor Prescription Fills Among 2014 Medicare Beneficiaries With Parkinson Disease Treated With an Acetylcholinesterase Inhibitor.
| Characteristic | Adjusted Odds Ratio (95%CI)a |
|---|---|
| Race/ethnicity | |
| White | 1 [Reference] |
| Black | 0.83 (0.77-0.89)b |
| Other/unknown | 0.95 (0.83-1.10) |
| Asian | 0.94 (0.83-1.06) |
| Hispanic | 1.11 (1.00-1.23)b |
| Native American | 0.99 (0.69-1.41) |
| Sex | |
| Male | 1 [Reference] |
| Female | 1.30 (1.25-1.35)b |
| Age, y | |
| 65-69 | 1 [Reference] |
| 70-74 | 0.86 (0.79-0.93)b |
| 75-79 | 0.75 (0.70-0.81)b |
| 80-84 | 0.66 (0.62-0.72)b |
| ≥85 | 0.57 (0.53-0.61)b |
| Comorbid disease (yes vs no)a | |
| Atrial fibrillation | 0.86 (0.81-0.90)b |
| Dementia | 1.76 (1.66-1.87)b |
| Depression | 1.62 (1.57-1.68)b |
| Diabetes | 1.07 (1.03-1.11)b |
| CKD | 1.08 (1.03-1.12)b |
| COPD | 1.12 (1.03-1.17)b |
| Heart failure | 1.06 (1.02-1.11)b |
| Hyperlipidemia | 0.98 (0.95-1.02) |
| Hypertension | 1.13 (1.09-1.19)b |
| Ischemic heart disease | 1.01 (0.97-1.05) |
| Stroke/TIA | 0.96 (0.91-1.01) |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack.
As measured using a logistic regression model that included race/ethnicity, age, sex, and (when applicable) indicator variable for the comorbid disease of interest.
Statistically significant estimate; a 2-sided P = .05 was considered statistically significant.
Geographic Variation in Parkinson Disease Never Events
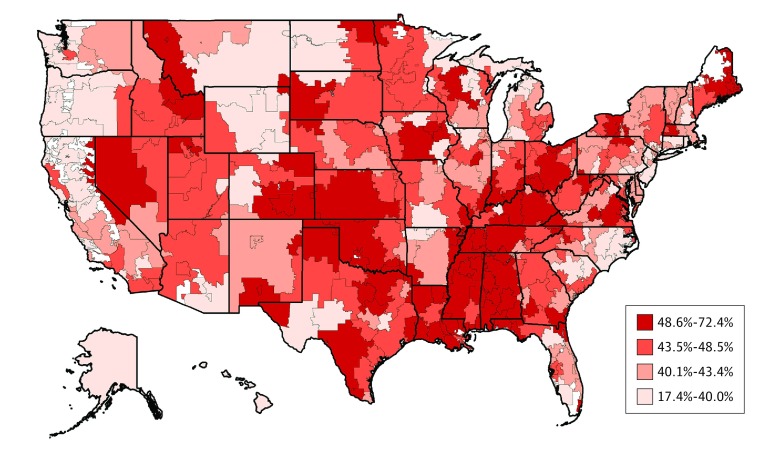
Wide geographic variation, from as low as 17.4% to as high as 72.4%, was observed in concurrent prescribing of a high-potency anticholinergic drug and an ACHEI among Medicare beneficiaries with Parkinson disease across hospital referral regions. As shown in the Figure, the prevalence of this never event demonstrated statistically significant spatial clustering (Moran I = 0.24; P < .001). Clusters of high prevalence of inappropriate prescribing were found in the southern and midwestern US regions, and clusters of low prevalence of inappropriate prescribing were present in the northwest and northeast US regions.
Figure. Percentage of 2014 Medicare Beneficiaries With Parkinson Disease Concurrently Using a High-Potency Anticholinergic Drug and an Acetylcholinesterase Inhibitor .
Discussion
An understudied threat to our ability to identify comprehensive and consistent factors in cognitive decline and dementia in Parkinson disease is the bias from cognitive impairment unrelated to Parkinson disease, that is, the disturbance or decline in cognition or performance on cognitive testing because of factors other than Parkinson disease progression. If random, such factors may mask the advantages of a neuroprotective medication; if systematic, spurious associations may be found or invalid risk factors for dementia or biomarkers associated with dementia may be identified. Of great concern are the preventable or reversible contributors to cognitive dysfunction, such as the adverse effects of medication.
In this study, we examined from a public health perspective the prescribing patterns associated with cognitive impairment in Parkinson disease. Our primary findings are (1) demographic differences exist in dementia drug choice and (2) inappropriate anticholinergic drug combinations, which can affect patient and research outcomes, are frequently prescribed to Medicare beneficiaries with a Parkinson disease diagnosis. These data call into question whether unique Parkinson disease trajectories across demographic groups exist, and the data suggest contributors to cognitive impairment in Parkinson disease that can be addressed at the population level.
We found that dementia treatment among individuals with Parkinson disease was prevalent, a finding consistent with the rising tide of dementia in the United States27 and with previous studies of dementia in Parkinson disease.5,28 We also found racial differences in the likelihood to receive dementia drugs prescription fill. Dementia medications were prescribed to black and Hispanic beneficiaries with Parkinson disease more often than for white beneficiaries. Hispanic individuals were given a prescription for memantine (a drug primarily indicated for severe disease) alone or in conjunction with an ACHEI more frequently than did individuals of other race/ethnicity. These observations have many possible explanations. Black Medicare beneficiaries with Parkinson disease are at greater risk than their white counterparts for both vascular dementia29 and Alzheimer disease.30 Thus, the observed increased odds of dementia medication use among black beneficiaries may be due to coexisting vascular dementia, Parkinson disease and dementia, or a mixed neurocognitive profile (eg, Parkinson disease and Alzheimer disease). The preferential use of memantine among Hispanic individuals in the sample suggest that this group may have a more severe form of dementia, present later in the disease course, or have greater intolerance of ACHEIs. Alternatively, lower cross-cultural validity of cognitive testing may overestimate dementia severity in some Hispanic people,31 particularly those for whom English is a second language. Implicit health care practitioner bias may drive the differences in prescribing or may magnify health disparities.32 Longitudinal studies of cognitive profiles among racial/ethnic groups with Parkinson disease are needed for a better understanding of the multiple factors in dementia treatment in Parkinson disease.
Women with Parkinson disease were less likely than men to be treated for dementia, even though they have a higher risk of clinical dementia because of the unrelated increased risk of Alzheimer disease in females.33 Dementia in women may be more sensitive to effect modification by comorbid disease treatment.34 Cognitive impairment may be under-recognized or undertreated in women, who are more likely to develop visuoconstructional deficits rather than impairments in activities of daily living and are less likely to receive specialist care.35,36 As with racial/ethnic minorities, sex-specific studies in Parkinson disease are needed.
ACHEIs and Anticholinergic Drug Concurrent Prescribing
Concurrent prescribing of a high-potency anticholinergic medication and an ACHEI, which should be a never event, was disturbingly common. Nearly 45% of Medicare beneficiaries with Parkinson disease and a clinical diagnosis of dementia filled a prescription for a medication that could further worsen their cognitive impairment in the course of a single year, assuming that the high-potency anticholinergic agent did not produce the cognitive impairment. We also identified several high-risk groups, which represent targets for immediate focus. Women and Hispanic people, who often receive lower-quality care and decreased access to care,37,38,39 were subject to this prescribing error more than were other groups. These findings are disturbing but not unique to the Parkinson disease population, as suggested by studies of prescribing error in antiretroviral therapy40 and antipsychotic therapy.41 Research designed to identify the factors in unsafe concurrent use and to define group-specific associations of drug-drug interactions may be needed to improve cognitive outcomes for all patients with Parkinson disease.
Comorbid diseases were associated with potentially inappropriate prescribing. In particular, depression was associated with 62% higher odds of potentially inappropriate drug combination prescribing. Several antidepressant medications, including tricyclic antidepressants and paroxetine, are classified as high-potency anticholinergic drugs. Alternatively, depressed mood and apathy are symptoms associated with cognitive decline, particularly in Parkinson disease,42 and are important to treat appropriately. Older adults frequently have multiple treatable medical diagnoses for which clinical guidelines recommend intermittent or chronic drug therapy. Thus, comorbid condition guidelines may be the initial intervention targets for reducing inappropriate prescribing in Parkinson disease. Future studies we are planning will need to include conditions that are not included in the CMS Chronic Conditions data set but are particularly relevant to patients with Parkinson disease, such as urinary tract dysfunction.
We found wide geographic variation in potentially inappropriate prescribing for Parkinson disease across the United States, ranging from 17.4% along the northwest and northeast coasts to as high as 72.4% in the Midwest and rural South. These geographic variances may be associated with local prescribing habits or may reflect differential access to neurologic care.36,43 In future studies, we will examine practitioner and practice factors in prescribing habits such as specialty, sex, patient volume, and care fragmentation. Regardless, our identification of the geographic disparities in prescribing safety raises the question of whether geography-based multidisciplinary interventions to improve practitioner education and patient outcomes, similar to the ParkinsonNet approach used in the Netherlands,44 would be effective in the United States.
Limitations
The CMS data set represents a national sample of US adults aged 65 years or older with a diagnosis of Parkinson disease and, to our knowledge, provides previously unreported information on inappropriate prescribing in this vulnerable patient population. However, some important limitations should be noted. We obtained claims data from a single year, which may not reflect current practice. The current data did not allow us to deeply examine the potential association of prescriber specialty and practice characteristics with the odds of dementia medication use and concurrent prescribing of a high-potency anticholinergic drug and an ACHEI. We also could not examine additional factors, such as out-of-pocket costs, that may affect prescription filling. These types of data are important for designing scalable interventions to reduce ineffective prescribing events.
Use of a dementia drug can be considered an indicator of cognitive impairment, but it is not an inclusive indicator of a dementia diagnosis. Thus, our drug use estimates do not reflect the prevalence of dementia among persons with Parkinson disease. Nondrug alternatives may be pursued at any stage of dementia for various reasons, including intolerance or response to nonmedical therapy. In addition, the study design did not allow us to examine beneficiary groups for which use of a dementia drug would be perceived as futile. Nursing home residents and individuals with proximately terminal conditions (eg, end-stage heart failure, metastatic cancer) may have an appropriate simplified medication regimen, which would prioritize weaning from medications (such as dementia drugs) that provide modest advantages over a long period. Use of off-label rivastigmine for freezing of gait45 or rapid eye movement behavior disorder46 has limited evidence support but may have occurred nonetheless. Drug prescribing errors are possible and cannot be traced in the CMS data set. All of these factors may affect our overall and demographic subgroup estimates of dementia medication use.
Some medications on the Anticholinergic Cognitive Burden Scale list are available without a prescription. We were unable to examine these medications in our prescription claim analysis; thus, our anticholinergic drug data may underestimate actual use. Future studies that include over-the-counter drug use (among other potential effect modifiers) may examine the associations between total anticholinergic burden or chronic anticholinergic exposure and clinical and research outcomes in people with Parkinson disease.
Conclusions
We identified unique patterns of dementia medication use among individuals with Parkinson disease, along with racial/ethnic, sex, and geographic disparities in potentially inappropriate prescribing habits. This nationwide assessment of dementia treatment in Parkinson disease in the United States may identify areas of particular concern for targeted study and intervention. In determining whether anticholinergic drug exposure has a causal role in clinical dementia in Parkinson disease, future studies may take a clinical trial approach, in which high-potency anticholinergic medications are replaced with lower-potency alternatives, and the change in cognitive testing and cognitive trajectory are measured. Such an approach will allow the calculation of anticholinergic drug safety in terms that are easily understood, such as number needed to harm. In addition, pragmatic approaches to practitioner education may lessen negative patient outcomes in the future.
eTable 1. Anticholinergic Burden Score
eTable 2. Demographic Characteristics Associated with Specific Dementia Drug Use Among Medicare Beneficiaries with Parkinson Disease, 2014
References
- 1.Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25(4):208-214. doi: 10.1177/0891988712464823 [DOI] [PubMed] [Google Scholar]
- 2.Heinzel S, Maechtel M, Hasmann SE, et al. Motor dual-tasking deficits predict falls in Parkinson’s disease: a prospective study. Parkinsonism Relat Disord. 2016;26:73-77. doi: 10.1016/j.parkreldis.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 3.Safarpour D, Thibault DP, DeSanto CL, et al. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413-419. doi: 10.1212/WNL.0000000000001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;22(suppl 1):S144-S148. doi: 10.1016/j.parkreldis.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 5.Reid WGJ, Hely MA, Morris JGL, Loy C, Halliday GM. Dementia in Parkinson’s disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry. 2011;82(9):1033-1037. doi: 10.1136/jnnp.2010.232678 [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72(6):708-712. doi: 10.1136/jnnp.72.6.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamikonyan E, Xie SX, Melvin E, Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: a placebo-controlled study. Mov Disord. 2015;30(7):912-918. doi: 10.1002/mds.26236 [DOI] [PubMed] [Google Scholar]
- 8.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509-2518. doi: 10.1056/NEJMoa041470 [DOI] [PubMed] [Google Scholar]
- 9.Hwang T-Y, Ahn I-S, Kim S, Kim DK. Efficacy of galantamine on cognition in mild-to-moderate Alzheimer’s dementia after failure to respond to donepezil. Psychiatry Investig. 2016;13(3):341-348. doi: 10.4306/pi.2016.13.3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvinenko IV, Odinak MM, Mogil’naya VI, Emelin AY. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial). Neurosci Behav Physiol. 2008;38(9):937-945. doi: 10.1007/s11055-008-9077-3 [DOI] [PubMed] [Google Scholar]
- 11.Campbell NL, Perkins AJ, Bradt P, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy. 2016;36(11):1123-1131. doi: 10.1002/phar.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papenberg G, Bäckman L, Fratiglioni L, Laukka EJ, Fastbom J, Johnell K. Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiol Aging. 2017;55:27-32. doi: 10.1016/j.neurobiolaging.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 13.Carrière I, Fourrier-Reglat A, Dartigues J-F, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317-1324. doi: 10.1001/archinternmed.2009.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi: 10.1111/j.1532-5415.2011.03491.x [DOI] [PubMed] [Google Scholar]
- 15.Uusvaara J, Pitkala KH, Kautiainen H, Tilvis RS, Strandberg TE. Detailed cognitive function and use of drugs with anticholinergic properties in older people: a community-based cross-sectional study. Drugs Aging. 2013;30(3):177-182. doi: 10.1007/s40266-013-0055-2 [DOI] [PubMed] [Google Scholar]
- 16.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401-407. doi: 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcum ZA, Wirtz HS, Pettinger M, et al. Anticholinergic medication use and falls in postmenopausal women: findings from the women’s health initiative cohort study. BMC Geriatr. 2016;16(1):76. doi: 10.1186/s12877-016-0251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sura SD, Carnahan RM, Chen H, Aparasu RR. Prevalence and determinants of anticholinergic medication use in elderly dementia patients. Drugs Aging. 2013;30(10):837-844. doi: 10.1007/s40266-013-0104-x [DOI] [PubMed] [Google Scholar]
- 19.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. [Google Scholar]
- 20.Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221(2):564-573. doi: 10.1016/j.bbr.2009.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdem E, Korda H, Haffer SC, Sennett C. Medicare claims data as public use files: a new tool for public health surveillance. J Public Health Manag Pract. 2014;20(4):445-452. doi: 10.1097/PHH.0b013e3182a3e958 [DOI] [PubMed] [Google Scholar]
- 22.Semla T, Beizer J, Higbee M. Geriatric Dosage Handbook: Including Clinical Recommendations and Monitoring Guidelines. Hudson, OH: Lexicomp Inc; 2006. [Google Scholar]
- 23.Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8-e18. doi: 10.1111/jgs.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Dartmouth Atlas of Health Care Research methods. http://www.dartmouthatlas.org/tools/faq/researchmethods.aspx. Accessed November 12, 2017.
- 25.Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143-151. doi: 10.1159/000275491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75(4):637-639. doi: 10.1136/jnnp.2003.020982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125-132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70(5):580-586. doi: 10.1001/jamaneurol.2013.2110 [DOI] [PubMed] [Google Scholar]
- 29.Gottesman RF, Fornage M, Knopman DS, Mosley TH. Brain aging in African-Americans: the Atherosclerosis Risk in Communities (ARIC) experience. Curr Alzheimer Res. 2015;12(7):607-613. doi: 10.2174/1567205012666150701102445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayegh P, Knight BG. Assessment and diagnosis of dementia in Hispanic and non-Hispanic white outpatients. Gerontologist. 2013;53(5):760-769. doi: 10.1093/geront/gns190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tensil M, Hessler JB, Gutsmiedl M, Riedl L, Grimmer T, Diehl-Schmid J. Sex differences in neuropsychological test performance in Alzheimer’s disease and the influence of the ApoE genotype. Alzheimer Dis Assoc Disord. 2018;32(2):145-149. doi: 10.1097/WAD.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 34.Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886-1893. doi: 10.1212/WNL.0000000000004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord. 2010;25(16):2695-2703. doi: 10.1002/mds.23388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77(9):851-857. doi: 10.1212/WNL.0b013e31822c9123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore JE, Mompe A, Moy E. Disparities by sex tracked in the 2015 National Healthcare Quality and disparities report: trends across national quality strategy priorities, health conditions, and access measures. Womens Health Issues. 2018;28(1):97-103. [DOI] [PubMed] [Google Scholar]
- 38.Gandhi K, Lim E, Davis J, Chen JJ. Racial-ethnic disparities in self-reported health status among US adults adjusted for sociodemographics and multimorbidities, National Health and Nutrition Examination Survey 2011-2014. Ethn Health. 2017;(November):1-14. doi: 10.1080/13557858.2017.1395812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia MA, Downer B, Chiu C-T, Saenz JL, Rote S, Wong R. Racial/Ethnic and Nativity Differences in Cognitive Life Expectancies Among Older Adults in the United States. Gerontologist. 2017;(September). doi: 10.1093/geront/gnx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Commers T, Swindells S, Sayles H, Gross AE, Devetten M, Sandkovsky U. Antiretroviral medication prescribing errors are common with hospitalization of HIV-infected patients. J Antimicrob Chemother. 2014;69(1):262-267. doi: 10.1093/jac/dkt323 [DOI] [PubMed] [Google Scholar]
- 41.Iversen TSJ, Steen NE, Dieset I, et al. Side effect burden of antipsychotic drugs in real life: impact of gender and polypharmacy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:263-271. doi: 10.1016/j.pnpbp.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 42.Chagraoui A, Boukhzar L, Thibaut F, Anouar Y, Maltête D. The pathophysiological mechanisms of motivational deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:138-152. doi: 10.1016/j.pnpbp.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 43.Saadi A, Himmelstein DU, Woolhandler S, Mejia NI. Racial disparities in neurologic health care access and utilization in the United States. Neurology. 2017;88(24):2268-2275. doi: 10.1212/WNL.0000000000004025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloem BR, Munneke M. Revolutionising management of chronic disease: the ParkinsonNet approach. BMJ. 2014;348:g1838. doi: 10.1136/bmj.g1838 [DOI] [PubMed] [Google Scholar]
- 45.Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(3):249-258. doi: 10.1016/S1474-4422(15)00389-0 [DOI] [PubMed] [Google Scholar]
- 46.Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov Disord. 2012;27(4):559-561. doi: 10.1002/mds.24909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Anticholinergic Burden Score
eTable 2. Demographic Characteristics Associated with Specific Dementia Drug Use Among Medicare Beneficiaries with Parkinson Disease, 2014