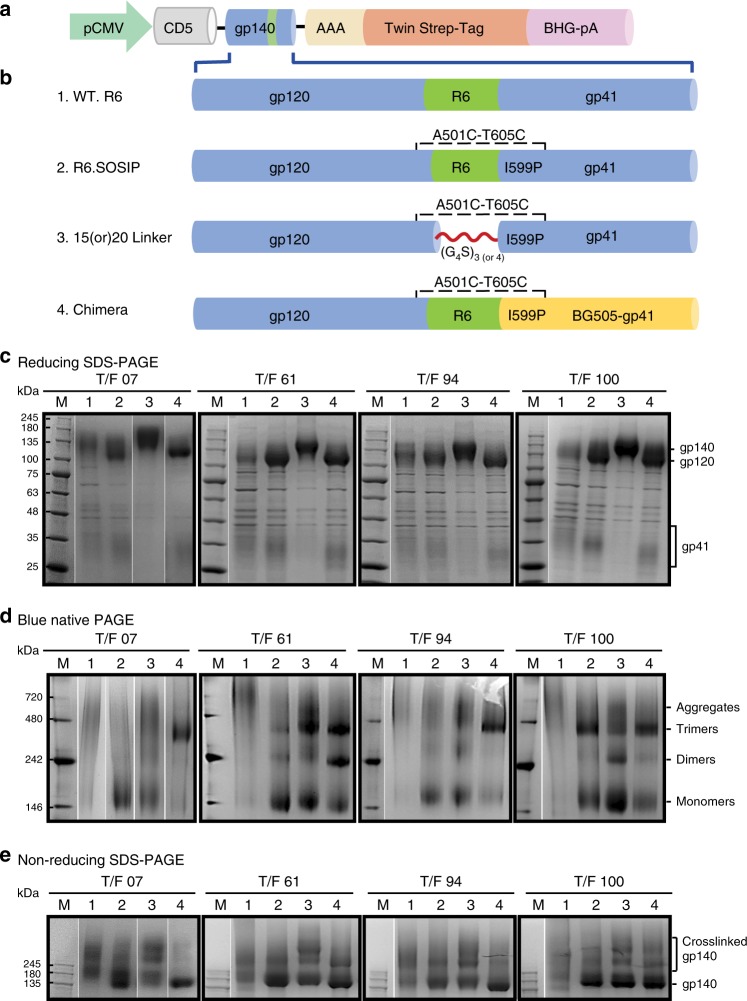
Fig. 1.
T/F gp140 recombinants predominantly produced gp140 monomers. a Schematic representation of gp140 Env expression vector. PCMV, CMV promoter; CD5, human CD5 signal peptide; Twin Strep-Tag, the 31-amino-acid sequence attached to the C-terminus of gp140; BGH-pA, 3′ poly-A sequence of bovine growth hormone gene. b Schematic of gp140 recombinant constructs. (1) WT. R6, wild-type construct with a furin protease cleavage site RRRRRR (R6) between gp120 and gp41. (2) R6. SOSIP, the three SOSIP mutations A501C, T605C, and I599P introduced into the WT.R6 clone. (3) 15 (or) 20 Linker, 15 (G4S)3 or 20 (G4S)4 amino acid linker introduced between gp120 and gp41 replacing the furin cleavage sequence. This construct also includes the SOSIP mutations. (4) Chimera, BG505 gp41 sequence replaced the gp41 of CRF01_AE T/F gp140 sequence. This construct also includes the R6 cleavage site and the SOSIP mutations. c–e The gp140s secreted into the culture medium were purified by Strep-Tactin affinity chromatography and analyzed by SDS polyacrylamide gel electrophoresis (PAGE) under reducing (c) or non-reducing (e) conditions, and by blue native (BN) PAGE (d), and stained with Coomassie Blue. All the R6-gp140s were cleaved by furin as shown by the appearance of gp120 and gp41 bands (c). The WT gp140 aggregated, producing nonspecific oligomers and essentially no trimers (or protomers) (d, lanes 1). All the SOSIP gp140s predominantly produced monomers except the T/F100 gp140, which produced both monomers and trimers (d, lanes 2). Linker insertion prevented furin cleavage and increased trimer production except in the case of the T/F100 gp140 (d, lanes 3). A major shift to trimers was observed in all the three Chimera gp140s; T/F07, T/F61, and T/F94, but no significant difference was observed with the T/F100 (d, lanes 4). The Linker and Chimera trimers (e, lanes 3 and 4) showed greater aggregation and nonspecific crosslinking than the trimers lacking either the linker or the BG505 gp41 chimera (e, lanes 2), as evident by the presence of higher oligomer species under nonreducing conditions (e, compare lanes 2 to lanes 3 and 4). The molecular masses in kDa for lanes M are shown on the left