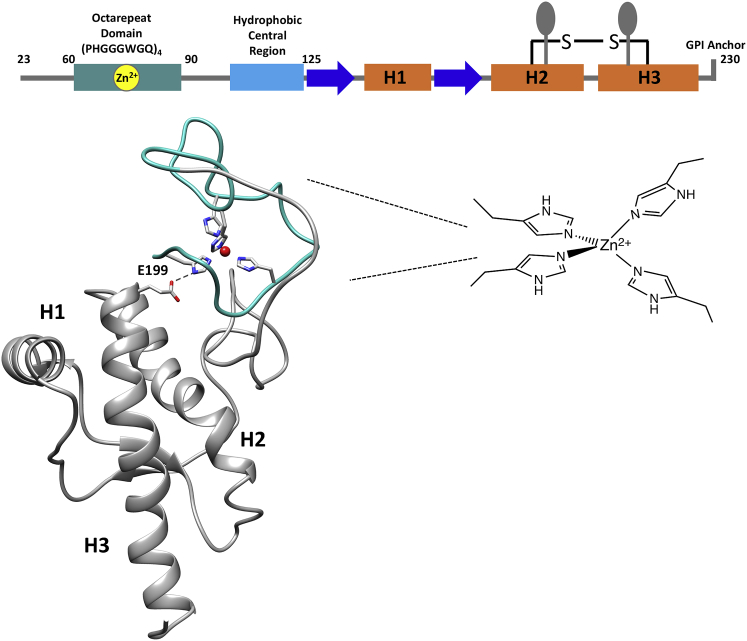
Figure 1.
Sequence and structural model of mouse PrPC-Zn2+ complex. (top) Linear sequence diagram of PrPC(23–230) (mouse sequence) with numbering indicating important N-terminal segments, along with secondary structure and posttranslational modifications of the folded C-terminal domain. Coloring shows the octarepeat (OR) domain (light green) that coordinates Zn2+, β sheets (blue), helices (orange), CR segment (light blue), glycans (ovals), disulfide bond linking helices two and three, and glycophosphatidylinositol anchor at residue 230. (bottom) Three-dimensional ribbon model of PrPC with Zn2+ (red sphere) coordinated to the OR domain His residues and docked against a C-terminal cleft formed by helices 1–3 (Spevacek et al. (15)). Also shown is residue E199 (mouse sequence) with its carboxylate group forming a hydrogen bond with the remote NH of a coordinating His residue.