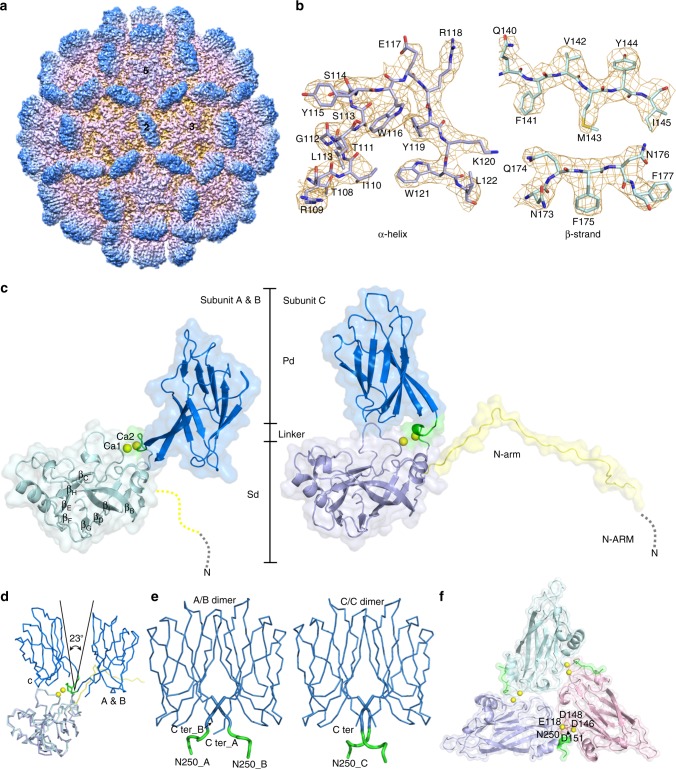
Fig. 1.
Cryo-EM asymmetric reconstruction of the T = 3 PvNV-LP at 3.5 Å resolution. a Front view of the cryo-EM map along an I2 symmetry axis with two, three, and five-fold axes as indicated. The capsid surfaces are five-colored according to their distances from the particle center (red: short distance; blue: long distance). b Segmented cryo-EM densities (orange mesh) with the corresponding atomic structures (sticks) of the typical α-helix and β-strands, respectively. c Ribbon diagrams and molecular surfaces of subunits A and B, and C with bound Ca2+ ions (yellow spheres), comprising several domains and regions, including N-ARM (gray), N-arm (yellow), the S-domain (light green and purple), the linker (green) and the P-domain (blue), respectively. d The different orientations of the subunits A and B, and C are color-coded as in c. e The homo-dimeric P-domains and linkers from the A/B and C/C dimers are color-coded as in c. Asn250 and the C-termini are indicated. f The S-domains of the three subunits per iASU. Six Ca2+ ions (yellow spheres) are incorporated at the interfaces of neighboring subunits A (light green), B (pink), and C (purple), where Glu118, Asp146, Asp148, Asp151, and Asn250 are involved in the Ca2+ binding