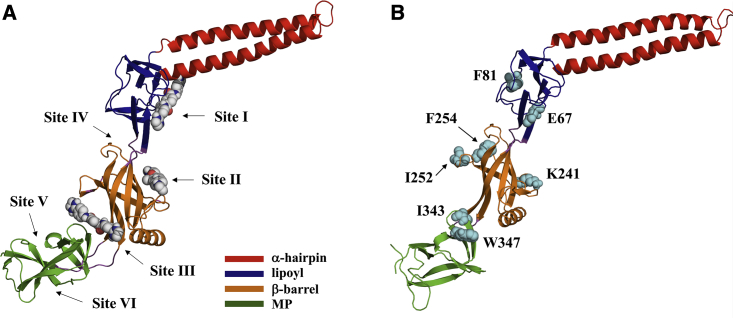
Figure 1.
(A) Inhibitor binding sites predicted from blind ensemble docking of SLU-258, clorobiocin, and novobiocin (sites I–III) and additional sites predicted by FTMap (sites IV–VI). Sites I, II, and III are located between the α-hairpin and lipoyl domains, in the β-barrel domain, and between the β-barrel and MP domain, respectively. Site IV is located between the β-barrel and lipoyl domain, and sites V and VI are located in the MP domain. For clarity, only SLU-258 is shown. (B) Residues mutated individually to Trp for fluorescence quenching experiments are labeled and shown as cyan spheres. The intrinsic Trp in wild-type AcrA, W347, was mutated to Phe. To see this figure in color, go online.