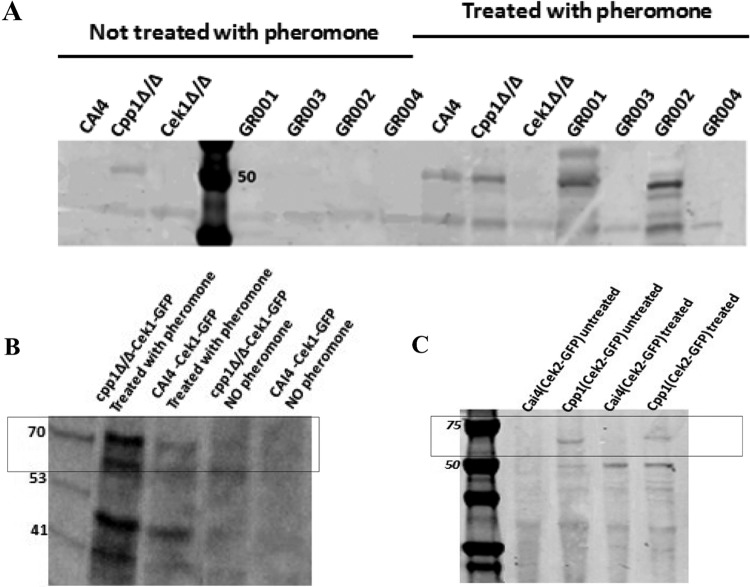
FIG 4.
Activation loop phosphorylation. Protein extracts from untreated strains and strains treated with α-pheromone were probed with an anti-phosphorylated-activation-loop antibody (anti-P44/42 MAPK polyclonal antibodies [Cell Signaling catalog no. 9101]) that detects the phosphorylated form of the Cek1 and Cek2 MAP kinases. (A) In the untreated lanes, only the strain lacking Cpp1 shows any phosphorylated MAP kinase; this band may be derived from phospho-Cek1 or -Cek2 because it is missing in the cpp1 mutant strains lacking either kinase. After pheromone treatment, the MAP kinase band shows enhanced phosphorylation in the WT strain; this appears to result from Cek1 because it is missing in all the cek1 deletion strains and enhanced in the cek2 deletion strain. This band is increased in the pheromone-treated Cpp1Δ/Δ strain and in the GR002 strains. To distinguish bands derived from Cek1 from those derived from Cek2, we examined the phosphorylation status of GFP fusions to the Cek1 and Cek2 kinases. (B) One allele of Cek1 is tagged with GFP in Cpp1Δ/Δ and CAI4 (WT) strains. Cek1-GFP activation site phosphorylation is enhanced by pheromone treatment and further enhanced by loss of the Cpp1 phosphatase. (C) Cek2 activation site phosphorylation is not detected in the untreated or the pheromone-treated WT strain but is enhanced equally in pheromone-treated or untreated cells lacking the Cpp1 phosphatase. (We loaded equivalent amounts of protein in each lane based on protein quantification by the Bradford assay.) Numbers in gels are molecular masses in kilodaltons.