Abstract
Aneurysmal bone cysts (ABC) are expansile lytic lesions constituting around 1% of all benign bone tumors with an annual incidence of 1.4/100000. A variety of treatments are available ranging from curettage with or without bone grafting (autologous or allogeneic), curettage with use of adjuvants [Polymethylmethacrylate (PMMA) bone cement, high speed burr, phenol, liquid nitrogen], wide en-block excision with or without reconstruction, selective arterial embolization of the feeding vessels, radiation therapy, high precision megavoltage radiotherapy and percutaneous radio-nuclide ablation, sclerotherapy (ethibloc, aetoxisclerol, alcohol gel, polidocanol). The optimal treatment is debatable due to various indications and contraindications of different modalities of treatment. Recent data suggest that percutaneous sclerotherapy with polidocanol is safe and effective alternative to surgery for treatment of ABCs as it has minimal side effects. We are reporting the first case of life-threatening adverse reaction to intra-lesional polidocanol in a three-year-old boy with a proximal femoral aneurysmal bone cyst. The importance of reporting this case is to make people aware regarding the adverse reaction of polidocanol and to highlight the precautions one should follow while using polidocanol for aneurysmal bone cysts.
Keywords: Polidocanol, Pediatric orthopedics, Aneurysmal bone cyst, Safety, Life-threatening
1. Introduction
Aneurysmal bone cysts (ABC) are expansile lytic lesions constituting around 1% of all benign bone tumors with an annual incidence of 1.4/100000.1,2 A variety of treatments are available ranging from curettage with or without bone grafting (autologous or allogeneic), curettage with use of adjuvants [Polymethylmethacrylate (PMMA) bone cement, high speed burr, phenol, liquid nitrogen], wide en-block excision with or without reconstruction, selective arterial embolization of the feeding vessels, radiation therapy, high precision megavoltage radiotherapy and percutaneous radio-nuclide ablation, sclerotherapy (ethibloc, aetoxisclerol, alcohol gel, polidocanol).3, 4, 5, 6 The optimal treatment is debatable due to various indications and contraindications of different modalities of treatment. Recent data suggest that percutaneous sclerotherapy with polidocanol is safe and effective alternative to surgery for treatment of ABCs as it has minimal side effects.7 We are reporting the first case of life-threatening adverse reaction to intra-lesional polidocanol in a three-year-old boy with a proximal femoral aneurysmal bone cyst.
2. Case history
In December 2015, a boy of three years presented to us with the complaints of pain in his left hip. On examination, there was tenderness over greater tuberosity. Hip movements were pain-free. Radiograph of left hip showed well defined expansile lytic lesion with multiple septae in femoral neck and trochanteric area with thinning of the cortex (Fig. 1). After detailed discussion with family and with written consent, we planned to do curettage with allogeneic bone grafting. The tissue obtained was sent for biopsy and it was positive for an aneurysmal bone cyst. Post-operatively, a hip-spica was given for six weeks. There were no immediate post-operative complications and the child was gradually mobilized, initially with support and full weight bearing was allowed after 10 weeks. Follow-up x-rays after 6 and 12 weeks suggested progressively increasing calcification at the site of the cyst (Fig. 2a and b). The cyst showed progressive signs of healing. Routine follow - up x-ray after one year depicted recurrence of the lytic lesion in the upper part of the previous lesion, although the patient was asymptomatic. At two years, x rays of his left hip (Fig. 3a and b) suggested further increase in the size of lesion and patient also started complaining dull aching pain in the affected hip. After discussing the prognosis with parents, percutaneous sclerotherapy with Polidocanol injection was planned. Parents were also counselled for possible repetition of instillation at around 12 weeks of the 1st injection.8 All routine blood investigations were within normal limits and patient had no known allergies. After pediatric and anesthesia clearance, the patient was taken for intralesional injection of Polidocanol under general anesthesia. Polidocanol is chemically hydroxy-poly-ethoxy-dodecan. It is available in 2 ml ampoules (SclerolR) and its 3% concentration is used for treating bone cyst. However, for treating venous malformations, its concentration is 1%.8
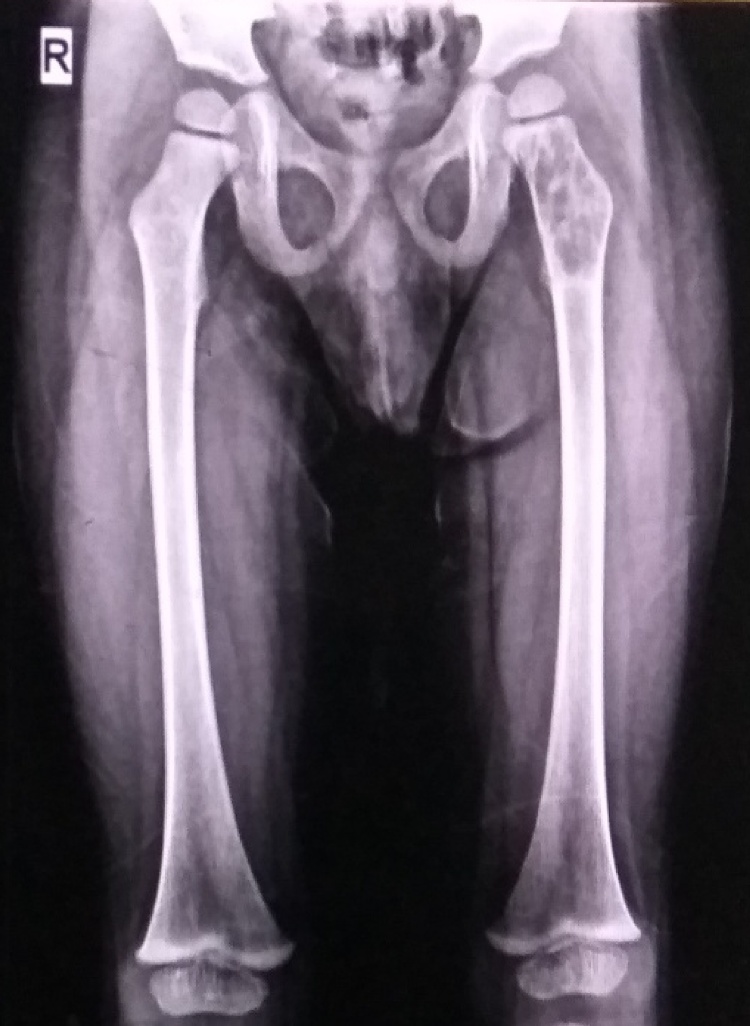
Fig. 1.
Antero-posterior radiograph showing expansile lytic lesion with thinning of cortex in left femur trochanteric and neck region.
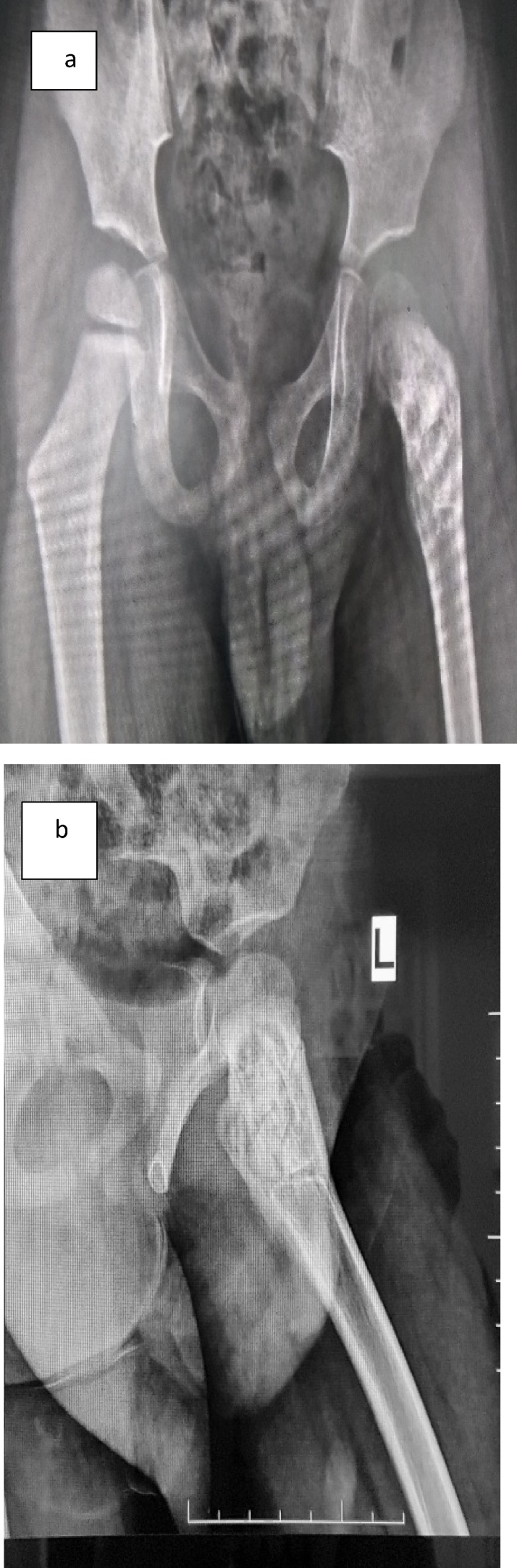
Fig. 2.
(a) Antero-posterior radiograph three months following extended curettage and bone grafting. (b) Frog lateral radiograph three months following extended curettage and bone grafting.
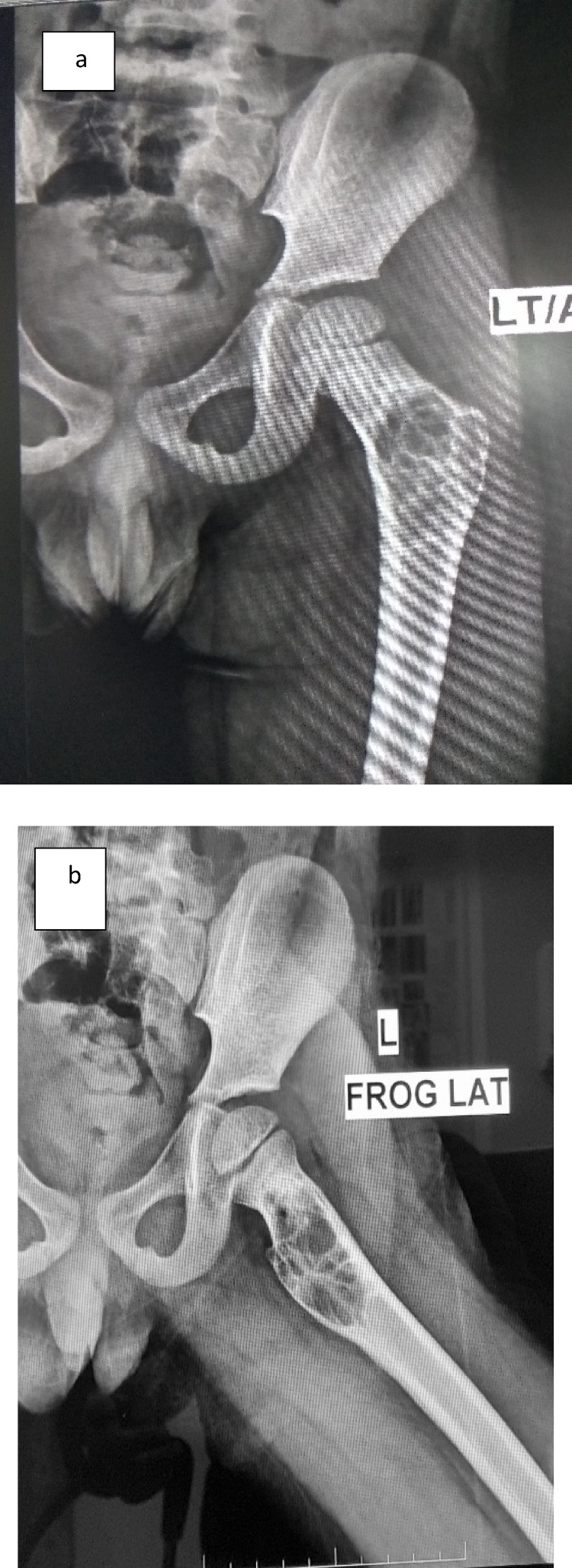
Fig. 3.
(a) Antero-posterior radiograph showing recurrence after two years post extended curettage and bone grafting at which time it was decided to instill intralesional Polidocanol. (b) Frog lateral radiograph of left femur showing recurrence of aneurysmal bone cyst after two years post extended curettage and bone grafting at which time it was decided to instill intralesional Polidocanol.
The volume of the cavity was calculated to be 18 cm3 and we planned to use 10 ml (the maximum dose which can be used per lesion per sitting) of 3% Polidocanol.8 Under c-arm guidance using an 18-gauge spinal needle, the cystic cavity was punctured and 20 ml of hemorrhagic fluid was aspirated. Initially, 2 ml of Polidocanol (to increase its area of contact, it was converted into froth) was slowly injected into the lesion. Within a minute, the patient developed fall in blood pressure from 96/52 mm of Hg to 80/30 mm of Hg and tachycardia (150 beats/min). We thought this to be a result of aspiration of 30 ml of fluid or use of froth containing Polidocanol. After waiting for 15 min for the child to stabilize (which he did) and with anesthetist’s approval, we slowly injected the remaining 8 ml of Polidocanol 9 without making froth). After a few minutes patient developed a sharp fall of blood pressure from 100/70 mm of Hg to 50/20 mm of Hg and increase in heart rate which soon turned into ventricular tachycardia with heart rate fluctuating between 150 to 200 beats per minutes with preserved pulses, though low volume (Ventricular Tachycardia with pulse). A pediatric cardiologist and pediatric intensivist were called in immediately. The child was started on amiodarone infusion 5 mg/kg and the rhythm reverted to sinus rhythm with occasional premature-ventricular complexes (PVC) half way through the dose. Gradually, heart rate came back to normal over a period of 20–30 min. The child was shifted to Pediatric ICU for monitoring where occasional ventricular ectopics were observed for next 24 h. The patient was discharged after 32 h with the intensivist’s clearance after he was normal and with a normal ECG.
3. Discussion
Polidocanol is a solvent and non-ionic emulsifier. Its injection has sclerosant and irritant property that causes the destruction of the endothelium of cyst or vasculature and causes thrombosis and occlusion of the cavity. Polidocanol is also used in ointments as local anesthetic and antipruritic agent. The various modalities of treatment of ABC have their own advantages and disadvantages. Recent literature supports the use of Polidocanol in ABC because of high healing rate and minimal complications compared to other modalities.7,8,10 It has been used and recommended to be used in an out-patient setting in view of its safety9. To our knowledge, not even a single study has reported such a severe life-threatening hypersensitivity reaction to the use of polidocanol in ABCs till date. Rastogi et al.8 had reported mild complications like induration at the injection site, hypopigmentation, local inflammatory reaction and dizziness. Batiste et al.11 had two cases of inflammatory reaction, one case of local erythema and one case of subcutaneous nodules. Other than ABC, Polidocanol is being used for a long time to treat vascular malformations. Guex12 treated nearly 11,000 cases of vascular malformation with Polidocanol and found only seven cases of minor urticaria, one case of cutaneous necrosis and some small epidermal necrosis but not even a single case of shock or severe adverse reaction.
4. Conclusion
Polidocanol instillation as a sclerosant in ABCs should not be taken lightly and should always be done in controlled environment preferably in operation theatre setting with the child intubated. Test dose must be given to ascertain any significant body reaction to the drug. It needs to be used very cautiously with one prepared to tackle any hypersensitive reaction. Its use in ABCs on an out-patient basis should be discouraged. Further larger and long-term studies are needed to establish its safety profile in ABCs.
Sources of support
Nil.
Presentation at a meeting
Nil.
Conflicts of interest
None.
Acknowledgement
None.
Contributor Information
Gaurav Gupta, Email: gauravgupta5038@gmail.com.
Ram Sagar Pandit, Email: ramsagarpandit7@gmail.com.
Nameet Jerath, Email: nameetjerath@yahoo.co.uk.
Ramani Narasimhan, Email: ramanirn@hotmail.com.
References
- 1.Cottalorda J., Gouin F. Aneurysmal bone cyst. In: Chotel F., Gouin F., editors. Benign osseous tumors. Elsevier Masson; Paris: 2005. pp. 188–200. [Google Scholar]
- 2.Topouchian V., Mazda K., Hamze B., Laredo J.-D., Pennecot G.-F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232:522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall W.M., Zlotecki R.A., Gibbs C.P., Reith J.D., Scarborough M.T., Mendenhall N.P. Aneurysmal bone cyst. Am J Clin Oncol. 2006;29(3):311–315. doi: 10.1097/01.coc.0000204403.13451.52. [DOI] [PubMed] [Google Scholar]
- 4.Cottalorda J., Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B. 2006;15(3):155–167. doi: 10.1097/01.bpb.0000210588.50899.29. [DOI] [PubMed] [Google Scholar]
- 5.Tomasik P., Spindel J., Miszczyk L. Treatment and differential diagnosis of aneurysmal bone cyst based on our own experience. Orthop Traumatol Rehabil. 2009;11(5):467–475. [PubMed] [Google Scholar]
- 6.Papagelopoulos P.J., Choudhury S.N., Frassica F.J., Bond J.R., Unni K.K., Sim F.H. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Jt Surg Am. 2001;83-A(11):1674–1681. doi: 10.2106/00004623-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Brosjö O., Pechon P., Hesla A., Tsagozis P., Bauer H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts Good results in 37 of 38 consecutive patients. Acta Orthopaedica. 2013;84(5):502–505. doi: 10.3109/17453674.2013.850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastogi S., Varshney M.K., Trikha V., Khan S.A., Choudhury B., Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J Bone Jt Surg (Br) 2006;88(9):1212–1216. doi: 10.1302/0301-620X.88B9.17829. [DOI] [PubMed] [Google Scholar]
- 9.Varshney M.K., Rastogi S., Khan S.A., Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop. 2010;468(6):1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosjo O., Tsagozis P. Treatment of an aggressive aneurysmal bone cyst with percutaneous injection of polidocanol: a case report. J Med Case Rep. 2014;8:450. doi: 10.1186/1752-1947-8-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batissea F., Schmittc A., Vendeuvrea T., Herbreteaub D., Bonnar C. Aneurysmal bone cyst: a 19-case series managed by percutaneous sclerotherapy. Orthop Traumatol: Surg Res. 2016;102:213–216. doi: 10.1016/j.otsr.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Guex J.J. Indications for the sclerosing agent polidocanol (aetoxisclerol dexo, aethoxisklerol kreussler) J Dermatol Surg Oncol. 1993;19:959–961. doi: 10.1111/j.1524-4725.1993.tb00985.x. [DOI] [PubMed] [Google Scholar]