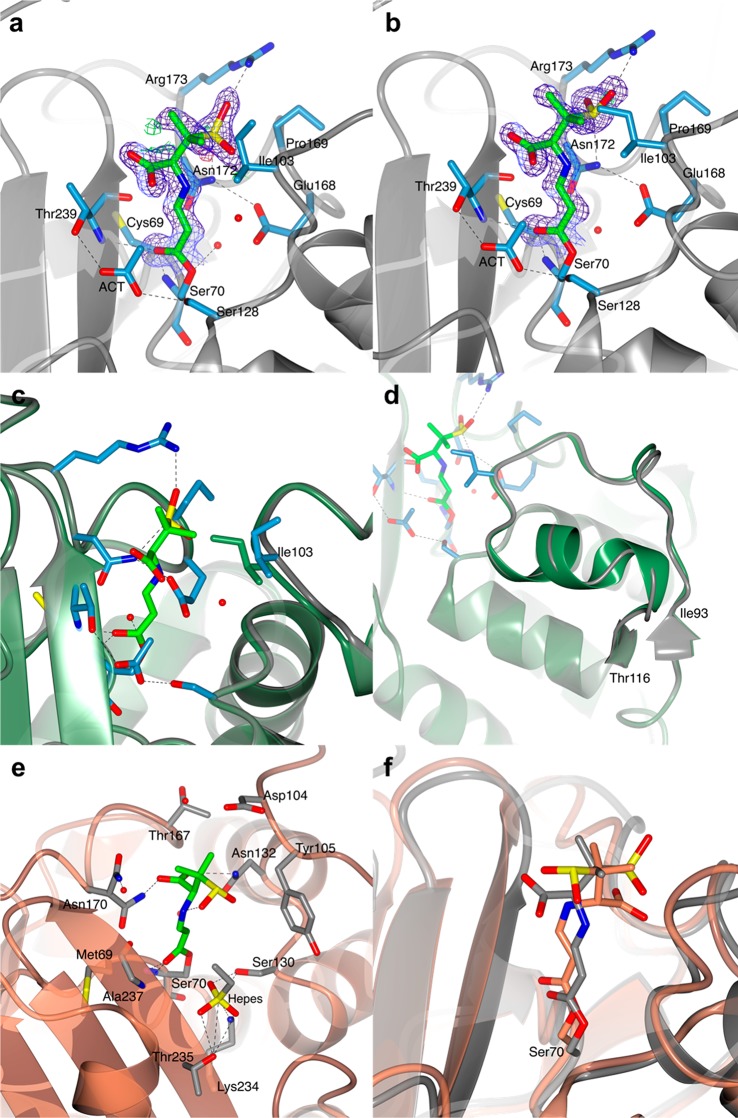
Figure 4.
Structure of the covalent trans-enamine adduct formed between sulbactam and BlaC. The modeled adducts in chains A (a) and B (b) of the AU are shown in stick representation colored according to atom type (C in green, O in red, N in blue, and S in yellow). The 2mFo-DFc electron density map (blue chicken wire with a contour level of 1 σ) is centered on the sulbactam adducts. Acetate ions (ACT) and protein residues at a hydrogen bond (dashed lines) distance from the adduct or at a distance < 4 Å are represented as sticks (C in light blue, O in red, and N in blue). Waters are represented as red spheres. BlaC is in ribbon representation (gray). (c and d) Superposition of BlaC in complex with the trans-enamine adduct of sulbactam (chain A, gray ribbon representation) and the structure of free BlaC (PDB 5OYO, lawn green). The presence of sulbactam in the active site of BlaC caused a slight shift of the region between residues 93–116, compared to the structure of free BlaC. (c) In chain A, sulbactam binding forced the side chain of Ile103 into a more open conformation to avoid clashes. (d) In chain B, sulbactam caused a destabilization of the α-helix made by residues 105–113. (e and f) Comparison of BlaC and SHV-1 covalent complexes with sulbactam. (e) Active site of SHV-1 bound to sulbactam (PDB 2A3U). The trans-enamine adduct is in stick representation and is colored according to atom type (C in green, O in red, N in blue, and S in yellow). SHV-1 fold is in ribbon representation (coral), and the residues involved in the stabilization of the inhibitor are represented as sticks (with C colored in gray). Hydrogen bonds are indicated as dashed lines. (f) Superposition of the X-ray crystal structures of BlaC (gray) and SHV-1 (PDB 2A3U, coral) in complex with sulbactam.