Abstract
The apical membrane Cl−/oxalate exchanger SLC26A6 has been demonstrated to play a role in proximal tubule NaCl transport based on studies in microperfused tubules. The present study is directed at characterizing the role of SLC26A6 in NaCl homeostasis in vivo under physiological conditions. Free-flow micropuncture studies revealed that volume and Cl− absorption were similar in surface proximal tubules of wild-type and Slc26a6−/− mice. Moreover, the increments in urine flow rate and sodium excretion following thiazide and furosemide infusion were identical in wild-type and Slc26a6−/− mice, indicating no difference in NaCl delivery out of the proximal tubule. The absence of an effect of deletion of SLC26A6 on NaCl homeostasis was further supported by the absence of lower blood pressure in Slc26a6−/− compared with wild-type mice on normal or low-salt diets. Moreover, raising plasma and urine oxalate by feeding mice a diet enriched in soluble oxalate did not affect mean blood pressure. In contrast to the lack of effect of SLC26A6 deletion on NaCl homeostasis, fractional excretion of oxalate was reduced from 1.6 in wild-type mice to 0.7 in Slc26a6−/− mice. We conclude that, although SLC26A6 is dispensable for renal NaCl homeostasis, it is required for net renal secretion of oxalate.
Keywords: chloride, oxalate, proximal tubule, SLC26A6
INTRODUCTION
SLC26A6 is an anion exchanger expressed in multiple tissues, including the kidney (12, 15, 20, 23). Several groups of investigators have demonstrated that SLC26A6 can mediate exchange of pairs of anions, including Cl−, , formate, and oxalate (2, 3, 8, 12, 28). Although a major fraction of Cl− reabsorption in the proximal tubule is passive and paracellular, tubule microperfusion studies have indicated the capability for a component of transtubular oxalate-dependent NaCl reabsorption in this nephron segment (21, 22, 25). Because SLC26A6 operates as a Cl−/oxalate exchanger (2, 8, 28) and is expressed on the brush-border membrane of the proximal tubule (12), it was postulated that SLC26A6 may be a major contributor to NaCl absorption in this nephron segment (1). Indeed, Cl−/oxalate exchange activity in brush-border membrane vesicles was abolished in membranes from Slc26a6−/− mice (7). Similarly, stimulation of NaCl absorption by oxalate in proximal tubules microperfused in situ was abolished in tubules of Slc26a6−/− mice (24). However, the role of SLC26A6 in renal NaCl homeostasis in vivo under physiological conditions is not known. It has also been speculated (17) that SLC26A6 may contribute to the active secretion of oxalate that has been observed in the proximal tubule (6, 18, 26). The purpose of this study is therefore to use Slc26a6−/− mice to investigate the roles of SLC26A6 in renal NaCl homeostasis and net renal secretion of oxalate.
MATERIALS AND METHODS
Animal studies.
Adult age-matched male or female 129S6/SvEv wild-type (WT) mice (Taconic, Rensselaer, NY, and Charles River Laboratories, Sulzfeld, Germany) and Slc26a6−/− mice on a congenic 129S6 background (7) were used. Micropuncture studies (Fig. 1) were performed using mice fed standard rodent chow (Envigo, Madison, WI). For diuretic clearance studies (Fig. 2), animals were fed an oxalate-free diet (TD 94045) after weaning. For telemetric blood pressure studies with varying sodium intake (Fig. 3), mice were fed commercial diets containing 1% NaCl (TD 90229) or sodium-deficient diets (TD 90228). For telemetric blood pressure studies with oxalate loading (Fig. 4), mice were fed an oxalate diet that was prepared by adding 10 μmol/g sodium oxalate to a calcium-free oxalate-free diet (TD 95027), as previously described (10, 16). For radiolabeled oxalate clearance studies (Fig. 5), mice were fed an oxalate-free diet (TD 97191). All animal protocols were approved by the Yale University Institutional Animal Care and Use Committee and by local regulatory authorities in Germany.
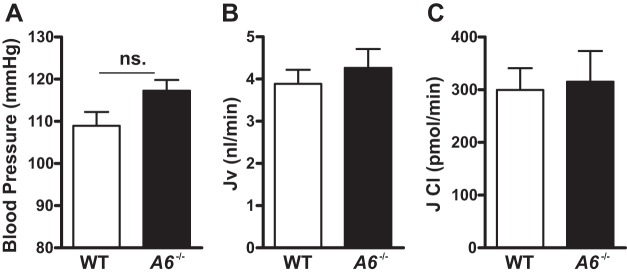
Fig. 1.
Mean blood pressure, proximal tubule volume, and Cl− absorption in male wild-type (WT) and Slc26a6−/− mice. A: male Slc26a6−/− mice have normal pressure. Proximal tubule volume (B) and Cl− absorption (C) as measured by free-flow micropuncture is similar between WT and Slc26a6−/− mice. ns, Not significant. Data are means ± SE from 9 mice in each group for blood pressure measurement, n = 18 for WT, and n = 19 for Slc26a6−/− for volume flux (Jv) and Cl measurement. P > 0.05 in Slc26a6−/− vs. WT mice for each group.
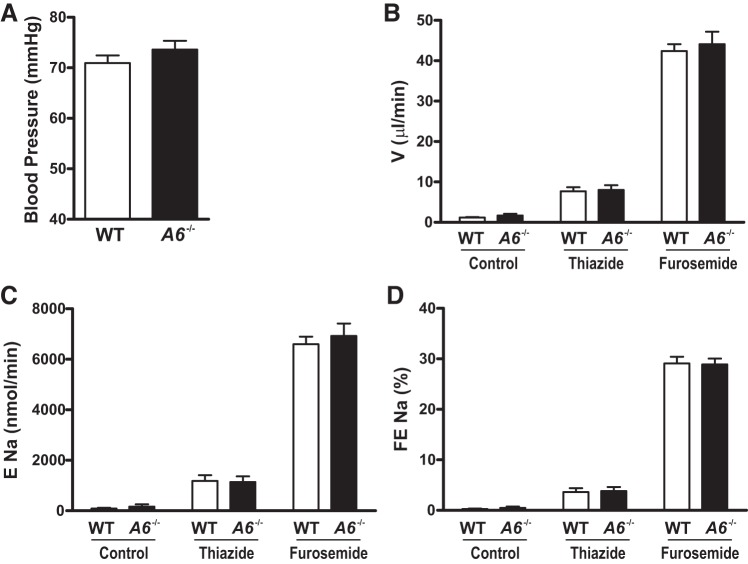
Fig. 2.
Mean blood pressure, urine flow rate, and sodium excretion following administration of hydrochlorothiazide (HCTZ) and furosemide in female wild-type (WT) and Slc26a6−/− mice. A: female Slc26a6−/− mice have normal pressure. Urine flow rate (B), sodium excretion (C), and fractional sodium excretion (D) are similar between WT and Slc26a6−/− mice following administration of thiazide and furosemide. Data are means ± SE from 7 mice in each group for blood pressure measurement and from 6 mice in each group for urine flow rate and fractional sodium excretion determination.
Fig. 3.
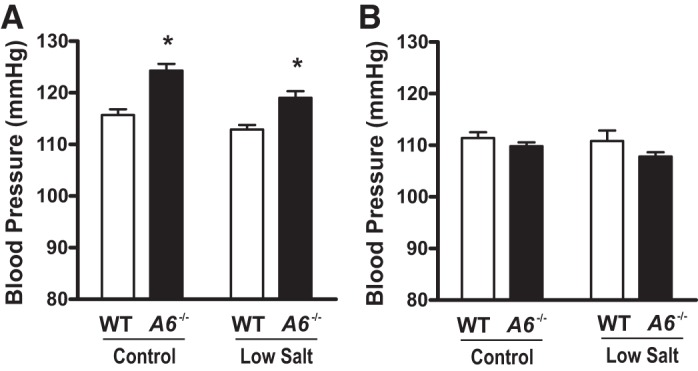
Ambulatory blood pressure of male and female wild-type (WT) and Slc26a6−/− mice on control and low-salt diet. A: male Slc26a6−/− mice have slightly elevated blood pressure on a control and low-salt diet compared with WT mice. B: female Slc26a6−/− mice have normal pressure on a control and low-salt diet. Data are means ± SE from n = 6 mice in each group for male mice and n = 7 for female mice in each group. *P < 0.05 in male Slc26a6−/− vs. WT mice on control and low-salt diet. P > 0.05 in female Slc26a6−/− vs. WT mice for each group.
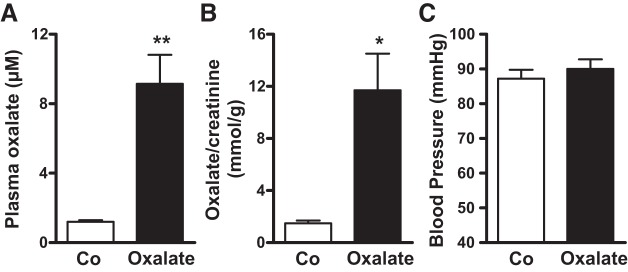
Fig. 4.
Mean blood pressure following feeding of a soluble oxalate diet. Plasma (A) and urine oxalate (B) concentrations are elevated in mice receiving a soluble oxalate diet vs. mice receiving an oxalate-free diet. C: mice receiving a soluble oxalate diet have no change in blood pressure. Data are means ± SE from 5 male mice for plasma and urine oxalate measurement and 4 male mice for blood pressure measurement. **P < 0.001 for plasma and *P < 0.05 for urine of mice receiving an oxalate diet vs. control diet. P > 0.05 for blood pressure measurement in mice receiving an oxalate diet vs. control diet.
Fig. 5.
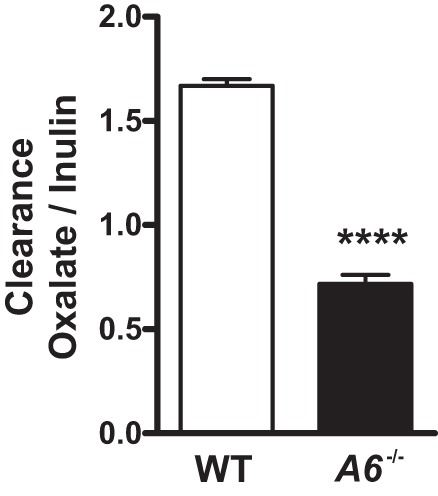
Mean oxalate-to-inulin clearance ratio in wild-type (WT) and Slc26a6−/− mice. Fractional excretion of oxalate is greatly reduced in Slc26a6−/− mice. Data are means ± SE from 2 male and 2 female mice in each group. ****P < 0.001 in Slc26a6−/− vs. WT mice.
Micropuncture studies.
Male WT and Slc26a6−/− mice had access to standard food and water ad libitum until the time of the experiment. Mice were anesthetized [125 mg/kg thiobutabarbital (Inactin, Sigma T-133; Merck, St. Louis, MO); 100 mg/kg ketamine IM (Merck)] and placed on a surgical table that maintained the body temperature near 37°C by circulating warmed water. The bladder was accessed via a midline incision in the lower abdominal area and catheterized (PE-50) to collect timed urine samples. An incision was made in the neck to access the trachea, common carotid artery, and external jugular vein. A tracheostomy was performed (pulled PE-240 tubing), and gentle flow of 100% oxygen was directed toward the open end of the tube. A catheter (pulled PE-50 tubing) filled with heparinized saline was inserted in the right carotid common artery to monitor and record blood pressure continuously and to obtain blood samples. Two PE-10 catheters were placed in the left jugular vein to infuse saline or a modified Ringer’s solution (in mM: 140 Na, 4 K, 115 Cl, 29 HCO3, bubbled with 5% CO2 and 2.25% BSA), [3H]inulin, and additional Inactin if necessary during the experiment. The left kidney was exposed via a flank incision and placed in a Lucite cup to isolate it from the respiratory movement of the animal according to standard micropuncture methods (19). The surface of the kidney was covered with warmed mineral oil and observed through a dissecting scope at ×160 magnification, and fluid samples were obtained from proximal tubules with sharpened glass micropipettes. The experiments lasted ~3 h. The animal was euthanized at the end of the experiment by giving an overdose of Inactin intravenously and by inducing a pneumothorax.
Random proximal tubules at the kidney surface were impaled with a glass pipette (tip outer diameter <5 µm) filled with a colored (FD&C Blue no. 1; H. Kohnstamm, New York, NY) saline solution. Injection of colored fluid in the proximal tubule permitted visualization and identification of the last surface proximal segment of the same nephron. A second glass pipette (tip outer diameter 10–12 µm) filled with stained (Sudan Black) mineral oil was used to inject an oil block at least four tubule diameters in length and then to make a complete timed fluid collection from the last or second to last surface proximal tubule segment. Samples were kept only if the fluid collection proceeded spontaneously and the position of the tubule oil block could be easily controlled after initiating the collection by applying a small amount of suction. After removing the tip of the pipette from the tubule upon termination of the timed collection, a small amount of the oil covering the kidney was aspirated in the tip of the pipette to prevent evaporation of the sample.
Volume of each sample was calculated by transferring the contents to a calibrated glass constant bore capillary tube and measuring the length of the fluid column. A portion of the sample was transferred to a liquid scintillation vial for counting of radioactivity ([3H]inulin; American Radiolabeled Chemicals, St. Louis, MO; Beckman LS600IC scintillation counter). The remaining sample was stored in the pipette between oil droplets until analyzed for Cl− concentration. Cl− was determined by electrometric titration (model F25; World Precision Instruments, Sarasota, FL, for tubule fluid; CM10, Radiometer America, Cleveland, OH, for plasma). Cl− concentration in plasma water was estimated by correcting measured values by 8% (multiplying by 1.08). Net proximal tubule fluid absorption Jv was calculated as the difference between single nephron glomerular filtration rate and the collected flow rate. Single nephron glomerular filtration rate was calculated as (TFIn/PIn) × TFV, where TFIn is the inulin concentration in collected tubule fluid, PIn is the inulin concentration in plasma and TFV is the rate of fluid collection at the late proximal site. Net Cl− transport was calculated as the difference between filtered and collected amounts.
Clearance studies.
Female WT and Slc26a6−/− mice raised on an oxalate-free diet were anesthetized (isoflurane 1–3% to effect in oxygen) and prepared surgically as above for the micropuncture studies except the kidney was not exposed. The experiments lasted ~4 h, and the animal was euthanized at the end of the experiment by pneumothorax under anesthesia. After the surgical preparation was complete, a 2.5% bolus of saline was injected intravenously, and the [3H]inulin infusion started at 18 µl·g−1·h−1. An equilibration period of 40 min was allowed to elapse before beginning timed urine collections under the following diuretic protocol: 30-min baseline collection, 30-min collection after infusion of 1% of body weight D5W, 20-min collection after injection of 5 mg/kg hydrochlorothiazide (Sigma H-4759; Merck), and 30-min collection after injection of 5 mg/kg furosemide (Sigma F-4381; Merck). The onset of diuresis after hydrochlorothiazide was gradual; however, the peak diuresis after furosemide occurred within the first 10 min after injection. Two further 30-min collections were obtained to monitor diuresis duration. [3H]inulin was infused at ~25–50 µCi/mouse over the course of the experiment. Blood samples (~20–50 µl) were taken at the midpoint of each urine collection, and plasma was saved. Radioactivity and sodium concentration (IL443 Flame Photometer; Instrumentation Laboratories) were measured in urine and plasma samples. Glomerular filtration rate was estimated as [3H]inulin clearance and was calculated as (UIn/PIn) × UV, where UIn is the inulin concentration in urine and UV is the urine flow rate. Fractional sodium excretion was calculated as the ratio between Na and inulin clearance.
Oxalate clearance studies were performed in male and female WT and Slc26a6−/− mice. Mice were fed an oxalate-free diet for 4 wk before study. Surgical preparation was as above for the diuretic studies. Approximately 75 µCi [3H]inulin and 10 µCi [14C]oxalate (Amersham Biosciences UK Limited, Buckinghamshire, UK) were infused per mouse over the course of the study. Saline infusion remained constant at 18 µl·g−1·h−1. After an initial equilibration period of 15–30 min, three 30-min urine collections were obtained with blood sampled at midpoints. Values obtained in the three collections were averaged for each mouse. The oxalate-to-inulin clearance ratio was calculated for WT and Slc26a6−/− mice.
Ambulatory blood pressure measurements.
Male and female WT and Slc26a6−/− mice were raised on an oxalate-free diet up until the time of the experiment and anesthetized with 1–3% isoflurane. Sterile surgical technique was observed throughout transmitter implantation, and ophthalmic ointment was applied to the eyes. A blood pressure transducer (TA11PA-C10 or PhysioTel TA11PA-C10; Data Sciences International, St. Paul, MN) was implanted in the left carotid artery, the body of the transducer was positioned subcutaneously on the right flank, and the skin was closed with surgical staples. The mice were subsequently given postoperative analgesics (0.3 mg/ml ibuprofen) in drinking water for 48 h and placed on the 1% NaCl control diet. Staples were removed after 7 days, and mice were singly housed and placed on a special receiver unit connected to a computer that monitors blood pressure from that mouse. Baseline data were observed for 3–5 days to ensure diurnal patterns disrupted by surgery had been reestablished, and, subsequently, the animals were placed on the sodium-deficient diet for 1 wk. A 3-day baseline period was used for analysis before switching diets. Days 3–5 were analyzed on the sodium-deficient diet. Twenty-four-hour mean ambulatory blood pressure on both diets was calculated. For oxalate loading, mice were maintained on a synthetic calcium- and oxalate-free diet (0% Ca, 0% oxalate) for 3 days, followed by soluble oxalate diet for 7 days (0% Ca, 0.134% sodium oxalate, both diets from Ssniff, Soest, Germany). Blood pressure was recorded for 7 days following transition to the soluble oxalate diet.
Oxalate measurements.
Urine and plasma oxalate was measured as previously reported (4). In brief, plasma and urine were filtered and acidified. Oxalate concentrations were measured enzymatically using oxalate oxidase (Trinity Biotech; Bray, Wicklow, Ireland) (4, 13).
Statistical analysis.
Statistical analysis was performed using the t-test for unpaired data. P < 0.05 was taken to indicate a significant difference.
RESULTS
Absence of SLC26A6 does not affect proximal tubule volume or Cl− absorption.
To assess the role of SLC26A6 in mediating volume and Cl− absorption in the proximal tubule under physiological conditions, free-flow micropuncture was performed. As shown in Fig. 1A, in these studies utilizing anesthetized animals, we detected no difference in blood pressure in Slc26a6−/− compared with WT mice. As shown in Fig. 1, B and C, volume and Cl− absorption were similar in surface proximal tubules of WT and Slc26a6−/− mice. Together, our findings suggest that, under physiological conditions, deletion of SLC26A6 does not have a detectable effect on NaCl reabsorption in proximal tubule segments accessible to micropuncture.
Absence of SLC26A6 does not affect delivery of NaCl out of the proximal tubule.
To evaluate the possibility that the role of SLC26A6 might be more evident when integrated across the whole length of proximal tubules of superficial and juxtamedullary nephrons, we next assessed NaCl delivery out of the proximal tubule by clearance studies. In these studies using anesthetized animals, we again detected no difference in blood pressure between Slc26a6−/− and WT mice as shown in Fig. 2A. We next sequentially administered hydrochlorothiazide and furosemide to assess the resulting increase in urine flow rate and sodium excretion as a measure of NaCl and fluid delivery out of the proximal tubule. As shown in Fig. 2, B–D, urine flow rate, sodium excretion, and fractional sodium excretion increased following administration of hydrochlorothiazide and furosemide. However, we detected no difference in these parameters between Slc26a6−/− and WT mice. These findings argue against an increased NaCl and fluid delivery out of the proximal tubule in Slc26a6−/− mice.
Ambulatory blood pressure is not decreased in Slc26a6−/− mice.
To further evaluate the possible role of SLC26A6 in NaCl homeostasis, we next placed mice on a control and low-salt diet. When extracellular fluid volume is reduced by restricting NaCl intake, a defect of proximal tubule NaCl reabsorption secondary to SLC26A6 deficiency might be expected to result in decreased blood pressure as found in Na/H exchanger isoform 3 null mice (14). However, as shown in Fig. 3, we did not observe lower blood pressure in Slc26a6−/− compared with WT mice on control or low-salt diet in either male (Fig. 3A) or female (Fig. 3B) animals. In fact, there was a slightly increased blood pressure in male Slc26a6−/− mice compared with WT mice. The absence of lower blood pressure in Slc26a6−/− compared with WT mice on control or low-salt diets further argues against a significant effect of SLC26A6 deletion on NaCl homeostasis.
Raising plasma and urine oxalate does not increase blood pressure.
The lack of effect of SLC26A6 deletion on NaCl absorption and blood pressure may have been secondary to low plasma and urine oxalate concentrations under the experimental conditions. Hence, we fed WT mice a soluble oxalate diet to increase plasma and urine oxalate concentrations to assess whether this would increase blood pressure. As shown in Fig. 4, A and B, feeding mice a diet of 0.134% sodium oxalate raised plasma and urine oxalate concentrations eightfold. However, this increase in plasma and urine oxalate concentrations was not accompanied by a change in blood pressure as shown in Fig. 4C. The finding that raising plasma and urine oxalate concentrations does not affect mean blood pressure provides additional evidence that oxalate-stimulated NaCl reabsorption is dispensable for renal NaCl homeostasis.
Oxalate clearance is greatly reduced in Slc26a6−/− mice.
As mentioned earlier, active secretion of oxalate has been observed in the proximal tubule (6, 18, 26), and it has been proposed that the SLC26A6 plays a role in this process (17). We therefore measured oxalate clearance in Slc26a6−/− compared with WT mice. Inulin clearance was not significantly different between Slc26a6−/− and WT mice (252 ± 16 vs. 298 ± 19 µl/min, respectively). As shown in Fig. 5, we confirmed previous findings that oxalate clearance exceeds inulin clearance, indicating net renal oxalate secretion (6, 18, 26). Most strikingly, the fractional excretion of oxalate decreased from 1.6 in WT mice to 0.7 in Slc26a6−/− mice, demonstrating that renal oxalate secretion is dependent on SLC26A6.
DISCUSSION
The main findings of the present study are that, under physiological conditions, SLC26A6 deletion does not have a detectable effect on renal NaCl homeostasis but ablates net renal oxalate secretion.
The conclusion that SLC26A6 deletion does not affect renal NaCl homeostasis is based on free-flow micropuncture studies showing that volume and Cl− absorption were similar in surface proximal tubules of WT and Slc26a6−/− mice; clearance studies demonstrating that the increments in urine flow rate and sodium excretion following thiazide and furosemide infusion were identical in WT and Slc26a6−/− mice, indicating no difference in NaCl delivery out of the proximal tubule; and the absence of lower blood pressure in Slc26a6−/− compared with WT mice on normal or low-salt diets. Moreover, raising plasma and urine oxalate by feeding mice a diet enriched in soluble oxalate did not affect mean blood pressure.
In fact, there was a tendency for blood pressure to be higher in Slc26a6−/− compared with WT mice, which reached statistical significance in the case of ambulatory blood pressure in male mice (Fig. 3). This difference in blood pressure is opposite to what one expects if there were a defect in renal tubule NaCl reabsorption in Slc26a6−/− mice. The mechanism underlying this blood pressure difference was not further evaluated in the present study.
The present findings that SLC26A6 deletion does not affect renal NaCl homeostasis stand in contrast to the previous demonstration using tubule microperfusion that oxalate-dependent NaCl absorption is abolished in Slc26a6−/− compared with WT mice (24). There are several possible explanations to explain these different results. First, studies with the use of microperfused tubules may not mimic physiological conditions in vivo. For example, substrates that compete for SLC26A6 transport activity under physiological conditions may be absent from the simple solutions used for tubule perfusion. Second, microperfusion of short proximal tubule segments in situ may not reflect the integrated transport processes observed over a longer length of the proximal tubule. Third, a defect in oxalate-stimulated NaCl reabsorption by SLC26A6-mediated Cl−/oxalate exchange may be compensated by NaCl reabsorption taking place by Cl−/formate exchange. Indeed, studies in brush-border membrane vesicles had suggested, based on substrate and inhibitor affinities, that Cl−/formate and Cl−/oxalate exchange occur by different transporters (9). Supporting this concept, brush-border membrane Cl−/formate exchange and formate-stimulated NaCl reabsorption were not significantly reduced in Slc26a6−/− compared with WT mice (7, 24). Nevertheless, the effects of formate and oxalate to stimulate NaCl reabsorption in microperfused tubule studies are not additive (22). Given that formate- and oxalate-dependent NaCl reabsorption are not additive, loss of either process alone may not result in a defect in proximal tubule NaCl reabsorption. Fourth, a defect in oxalate-stimulated NaCl reabsorption by SLC26A6-mediated Cl−/oxalate exchange may be compensated by passive NaCl reabsorption. Indeed, mathematical modeling has suggested that an increase in transcellular Cl− absorption induced by apical membrane Cl−/anion exchange may be traded off against passive paracellular Cl− transport by reducing the driving force for the latter, resulting in no net increase in Cl− absorption (27). This offsetting phenomenon may not have been observed in the previous microperfusion studies in which high luminal Cl− perfusate was used (24).
Although our studies did not reveal a detectable effect of SLC26A6 deletion on renal NaCl homeostasis, we did demonstrate for the first time that SLC26A6 is required for proximal tubule oxalate secretion. It had long been known that there is net oxalate secretion in the proximal tubule (6, 18, 26). The role of SLC26A6 to mediate epithelial oxalate secretion in the kidney is similar to its previously demonstrated role to mediate oxalate secretion in the intestine (5, 7, 11). Renal and intestinal oxalate secretion both function to eliminate oxalate from the body and thereby to lower the plasma oxalate concentration and reduce the total body burden of oxalate. However, whereas the role of SLC26A6-mediated oxalate secretion in the intestine has the effect of limiting net absorption of dietary oxalate and reducing urinary oxalate (5, 7), SLC26A6-mediated oxalate secretion in the kidney might be expected to raise urinary oxalate. Future studies using tissue-specific deletion of SLC26A6 will be needed to examine the contrasting roles of intestinal and renal SLC26A6 in oxalate homeostasis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Disease Grant R37-DK-033793 and the George M. O’Brien Kidney Center at Yale (P30-DK-079310) to P. S. Aronson, the Deutsche Forschungsgemeinschaft (KN 1148/2-1 and CRC 1365 Renoprotection) and Oxalosis- and Hyperoxaluria Foundation to F. Knauf, and TRENAL program by the German Academic Exchange Service to V. Pfann.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.S.A. and H.V. conceived and designed research; F.K., H.V., V.P., and Z.J. performed experiments; F.K., H.V., V.P., Z.J., and P.S.A. analyzed data; F.K., H.V., V.P., Z.J., and P.S.A. interpreted results of experiments; F.K., H.V., V.P., Z.J., and P.S.A. prepared figures; F.K., H.V., V.P., Z.J., and P.S.A. drafted manuscript; F.K., H.V., V.P., Z.J., and P.S.A. edited and revised manuscript; F.K., H.V., V.P., Z.J., and P.S.A. approved final version of manuscript.
REFERENCES
- 1.Aronson PS. Essential roles of CFEX-mediated Cl(-)-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis. Kidney Int 70: 1207–1213, 2006. doi: 10.1038/sj.ki.5001741. [DOI] [PubMed] [Google Scholar]
- 2.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005. doi: 10.1074/jbc.M411703200. [DOI] [PubMed] [Google Scholar]
- 3.Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL. Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J Physiol 586: 1291–1306, 2008. doi: 10.1113/jphysiol.2007.143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ermer T, Kopp C, Asplin JR, Granja I, Perazella MA, Reichel M, Nolin TD, Eckardt KU, Aronson PS, Finkelstein FO, Knauf F. Impact of regular or extended hemodialysis and hemodialfiltration on plasma oxalate concentrations in patients with end-stage renal disease. Kidney Int Rep 2: 1050–1058, 2017. doi: 10.1016/j.ekir.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 6.Greger R, Lang F, Oberleithner H, Deetjen P. Handling of oxalate by the rat kidney. Pflugers Arch 374: 243–248, 1978. doi: 10.1007/BF00585601. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 9.Karniski LP, Aronson PS. Anion exchange pathways for Cl− transport in rabbit renal microvillus membranes. Am J Physiol Renal Fluid Electrolyte Physiol 253: F513–F521, 1987. doi: 10.1152/ajprenal.1987.253.3.F513. [DOI] [PubMed] [Google Scholar]
- 10.Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84: 895–901, 2013. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011. doi: 10.1681/ASN.2011040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98: 9425–9430, 2001. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladwig PM, Liedtke RR, Larson TS, Lieske JC. Sensitive spectrophotometric assay for plasma oxalate. Clin Chem 51: 2377–2380, 2005. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 14.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol 281: F718–F727, 2001. doi: 10.1152/ajprenal.2001.281.4.F718. [DOI] [PubMed] [Google Scholar]
- 15.Lohi H, Kujala M, Kerkelä E, Saarialho-Kere U, Kestilä M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70: 102–112, 2000. doi: 10.1006/geno.2000.6355. [DOI] [PubMed] [Google Scholar]
- 16.Mulay SR, Eberhard JN, Pfann V, Marschner JA, Darisipudi MN, Daniel C, Romoli S, Desai J, Grigorescu M, Kumar SV, Rathkolb B, Wolf E, Hrabě de Angelis M, Bäuerle T, Dietel B, Wagner CA, Amann K, Eckardt KU, Aronson PS, Anders HJ, Knauf F. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am J Physiol Renal Physiol 310: F785–F795, 2016. doi: 10.1152/ajprenal.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robijn S, Hoppe B, Vervaet BA, D’Haese PC, Verhulst A. Hyperoxaluria: a gut-kidney axis? Kidney Int 80: 1146–1158, 2011. doi: 10.1038/ki.2011.287. [DOI] [PubMed] [Google Scholar]
- 18.Senekjian HO, Weinman EJ. Oxalate transport by proximal tubule of the rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 243: F271–F275, 1982. doi: 10.1152/ajprenal.1982.243.3.F271. [DOI] [PubMed] [Google Scholar]
- 19.Vallon V. Micropuncturing the nephron. Pflugers Arch 458: 189–201, 2009. doi: 10.1007/s00424-008-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldegger S, Moschen I, Ramirez A, Smith RJ, Ayadi H, Lang F, Kubisch C. Cloning and characterization of SLC26A6, a novel member of the solute carrier 26 gene family. Genomics 72: 43–50, 2001. doi: 10.1006/geno.2000.6445. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Egbert AL Jr, Abbiati T, Aronson PS, Giebisch G. Mechanisms of stimulation of proximal tubule chloride transport by formate and oxalate. Am J Physiol Renal Physiol 271: F446–F450, 1996. doi: 10.1152/ajprenal.1996.271.2.F446. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Giebisch G, Aronson PS. Effects of formate and oxalate on volume absorption in rat proximal tubule. Am J Physiol Renal Physiol 263: F37–F42, 1992. doi: 10.1152/ajprenal.1992.263.1.F37. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/ exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 25.Wareing M, Green R. Effect of formate and oxalate on fluid reabsorption from the proximal convoluted tubule of the anaesthetized rat. J Physiol 477: 347–354, 1994. doi: 10.1113/jphysiol.1994.sp020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinman EJ, Frankfurt SJ, Ince A, Sansom S. Renal tubular transport of organic acids. Studies with oxalate and para-aminohippurate in the rat. J Clin Invest 61: 801–806, 1978. doi: 10.1172/JCI108994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein AM. Chloride transport in a mathematical model of the rat proximal tubule. Am J Physiol Renal Physiol 263: F784–F798, 1992. doi: 10.1152/ajprenal.1992.263.5.F784. [DOI] [PubMed] [Google Scholar]
- 28.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]