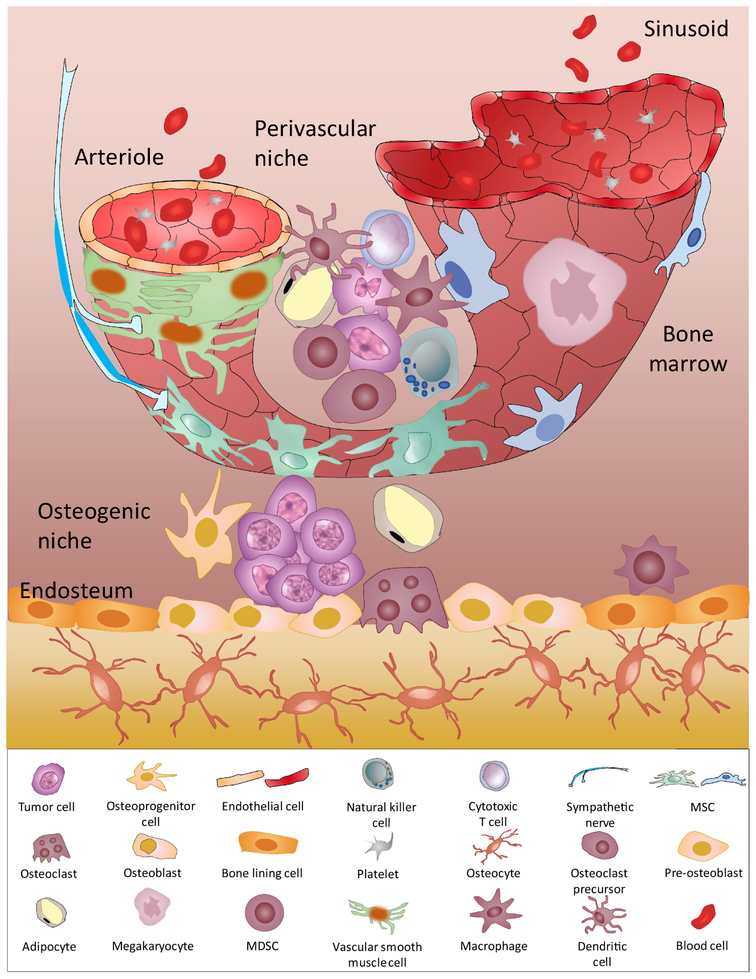
Figure 3, Key Figure Bone Marrow Niches for Metastatic Tumor Cells.
Generally, two different bone marrow niches may host tumor cells. Osteogenic niches (or endosteal niches) are adjacent to the endosteum, which are comprised mainly of osteoblastic cells. Our work suggested that osteogenic cells establish physical connection with residing tumor cells through heterotypic adheren junctions and gap junctions. This interaction activates the mTOR pathway in cancer cells and triggers calcium influx from niche cells, which promotes cancer cell survival and proliferation. By contrast, the perivascular niche is proposed to be a dormancy permissive niche. TSP-1 from endothelial cells maintains tumor cells in a dormant status. Other stromal cells in the perivascular niche are highly heterogenous, including MSCs expressing NG2+, Nes-GFP+, LepR+, or CXCL12 abundant reticular cells (CAR). As such, the perivascular niches may exert complex effects on residing tumor cells. Given that the endosteum is also highly vascularized, it is plausible that osteogenic niches and perivascular niches may spatially overlap in the endosteal region. In addition, MSCs can undergo osteogenic differentiation to generate osteoblastic lineage in vivo. Other cells types found in the bone marrow, including lymphocytes, MDSCs, macrophages, adipocytes, osteoclasts, megakaryocytes, and sympathetic nerves, can directly and indirectly participate in these two niches to orchestrate the progression of bone metastasis.