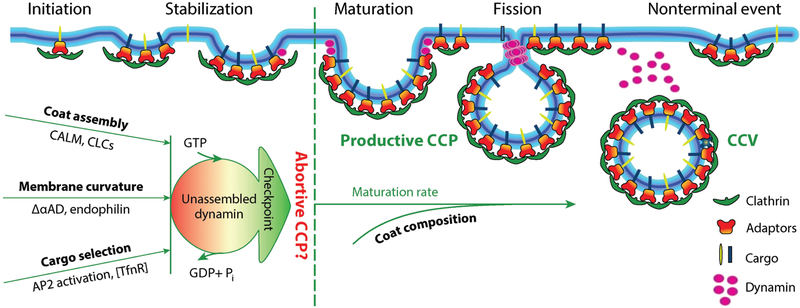
Figure 3.
The endocytic checkpoint hypothesis. A hypothetical endocytic checkpoint monitors the fidelity of CCP maturation. Deficiencies in several endocytic accessory proteins lead to decreased efficiency of CCP maturation and increased rates of turnover of abortive CCPs. Small interfering RNA-mediated knockdown of dynamin-2 decreases the rate of abortive CCP turnover. Based on these data, we speculate that the properties of nascent CCPs, such as coat assembly, curvature generation, and cargo concentration, are monitored by SH3 domain-containing dynamin binding partners that also interact with coat components, cargo, and/or sense curvature and kinetically control dynamin assembly. Together, these interactions function to monitor the fidelity of CCP maturation. Much remains to be done to test this hypothesis and to identify components of the endocytic checkpoint apparatus and the mechanisms to sense and turnover aberrant CCP intermediates. Abbreviations: AD, appendage domain; AP2, adaptor protein-2; CALM, clathrin assembly lymphoid myeloid leukemia; CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; CLCs, clathrin light chains; SH3, Src-homology domain 3; TfnR, transferrin receptor. Figure modified from Schmid (107).