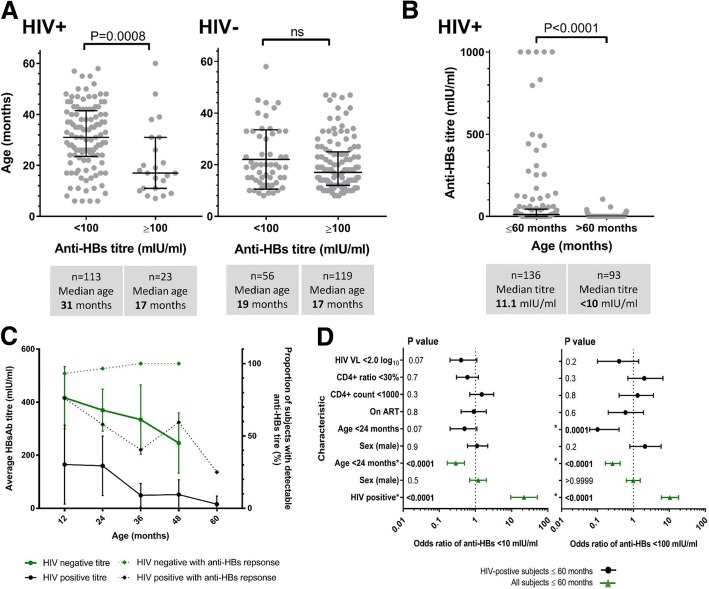
Fig. 3.
Relationship between age and vaccine-mediated hepatitis B surface antibody (anti-HBs) titres in HIV-positive and HIV-negative children in Kimberley, South Africa. A Ages of children attaining anti-HBs titres ≥ 100 mIU/ml for HIV-positive and HIV-negative children age 6–60 months. Median ages, interquartile ranges, and p values by Mann-Whitney U test are indicated. B Relationship between age and vaccine-mediated Ab titre among HIV-positive children including those age 6–60 months and an older cohort age > 60 months (range 64–193 months). p value by Mann Whitney U test. C Anti-HBs titre and proportion of subjects with a detectable titre for HIV-positive and HIV-negative children according to age. On the solid lines, each point represents the mean titre (with 95% confidence intervals) for the group of children aged ≤ 12 months (1 year), 13–24 months (2 years), 25–36 months (3 years), 37–48 months (4 years), and 49–60 months (5 years). For the same groups of children, the dotted lines represent the proportion of subjects with a detectable titre. Trends within the data were assessed using linear regression analysis. D Odds ratios for protective response to HBV vaccination in children age 6–60 months in Kimberley, South Africa, are shown for anti-HBs titre < 10 mIU/ml and < 100 mIU/ml in the whole cohort (green) and in HIV-positive children (black). Statistically significant OR are denoted by asterisk and significant p values are indicated in bold