Abstract
Background:
Sleep bruxism (SB) in children is commonly a self-limited problem; however, therapy of the condition may be needed to improve sleep quality of parents and children. Benzodiazepines have some success in controlling adult bruxism. The objective of this study was to evaluate the effect and the safety of a short course of diazepam on controlling SB in healthy children.
Materials and Methods:
In this double-blind, randomized placebo-controlled clinical trial, 109 children with SB were randomly assigned to three groups, receiving low or moderate dose of diazepam or placebo for 2 weeks. For children aged 2–8 years, the dose of 2.5 and 5 mg was considered as low and moderate dose consequently. In children >8 years, the doses were doubled. The severity of SB was evaluated at the beginning and also 2, 8, and 12 weeks thereafter. Data were collected by a questionnaire completed by parents including frequency of SB per week and per night and duration of each SB, as bruxism severity score (BSS). A mixed-model ANOVA was used to assess the differences of mean BSS between different groups and measurement times.
Results:
From 109 children recruited, 90 completed the study. After 2 weeks of intervention, the mean BSS decreased significantly in all groups (P = 0.0001), but it was not significantly different between groups in any of follow-ups (P = 0.554). Next-day sleepiness was assessed at week 2 of the study and was significantly higher in the groups using diazepam (P = 0.026).
Conclusion:
Short course of diazepam was not more effective than placebo for long-term control of SB in children.
Keywords: Benzodiazepines, child, pharmacological treatment, sleep bruxism
INTRODUCTION
Bruxism is a repeated movement of masticatory muscles characterized by grinding or clenching of the teeth and/or by forcing or bracing of the mandible. It may occur during sleep or wakefulness. “Probable case” needs the inspection of bruxism by examiner, while “definite one” necessities confirmation by polysomnographic and preferably video/audio recordings.[1] The reported prevalence of bruxism is so variable in children and ranges from 3.5% to 40.6% in different studies. Studies commonly report a decrease in the prevalence of sleep bruxism (SB) with age, and there are no gender differences.[2]
Although the condition is presumed as a benign and self-limited state in children, when exacerbated, it can cause disturbance of orofacial structures.[3] Another consequence is sleep disturbances of parents due to grinding sounds.
The etiology of SB is still unknown. Some aspects including tooth interference in dental occlusion, anxiety or stress, and genetic causes might be attributed.[3]
Despite the high occurrence of SB and its effects during childhood, there are limited studies related to the management of the condition in children. Options with limited success include oral devices, pharmacological agents, and cognitive behavioral techniques.[4]
The pharmacologic agents such as analgesics, anti-inflammatories, muscle relaxants, benzodiazepines, precursors of catecholamines, and beta-adrenergic antagonists were studied in the adult population with some successes. However, there is not any definite first-choice drug with enough safety and efficacy to be used in children.[4] Therefore, it is important to design appropriate studies to evaluate the effect of safe therapeutic agents to control underlying causes in severe episodes of SB in children.
Benzodiazepines are a class of drugs with anxiolytic and antispasmodic properties which had been used for the treatment of severe bruxism in adult population. In two randomized, double-blind studies in the adult population, administrating 1 mg single dose of clonazepam before sleep significantly reduced the bruxism index.[5,6] The experts’ opinion in our region suggests that consumption of oral diazepam for only 7–14 days is safe and might have short-term and long-term effects in reducing severe attacks.
Benzodiazepines are being used in clinical practice from many years ago in adult and children populations because of their effectiveness and safety. Prescriptions have decreased with awareness about potential adverse effects with prolonged use of the agents, including depression, tolerance, dependency, withdrawal, and cognitive deficits. However, short-term medication use (<6–12 weeks) might be safe in both adult and pediatric populations.[7,8]
Diazepam is a medium-potency, long-acting benzodiazepine that is used for treating a range of conditions, including anxiety, muscle spasms, seizures, and sleep disorder. Anxiolytic effects of the drug are seen at low doses. At higher doses, myorelaxation in addition to anxiolytic effects may be observed.[7]
Our study hypothesis is that a short-term (<14 days) course of diazepam might reduce proxy-reported SB for long time. Therefore, we decided to conduct a trial on the effects and safety of short course of diazepam in children with SB.
MATERIALS AND METHODS
Patients and settings
This was a double-blind, placebo-controlled, parallel three-arm randomized clinical trial (RCT). It was performed from July 2015 to April 2016 on children referred to the Imam Hossein Children Teaching Hospital, affiliated to the Isfahan University of Medical Sciences, Isfahan, Iran.
Inclusion criteria were as follows: (1) age of 2–15 years and (2) any frequency of bruxism reported by parents. Exclusion criteria were (1) inability to take a pill or a crushed pill, (2) significant drowsiness and vertigo, (3) drug hypersensitivity, (4) history of seizure disorder or abnormal movement during sleep, (5) any chronic oropharyngeal, respiratory, neurologic, and cardiac disturbance such as dental malocclusion, asthma, adenoid or tonsillar hyperplasia, nasal obstruction, cerebral palsy, and cyanotic heart disease, and (6) taking any medications affecting neuromuscular system, such as anticonvulsants, benzodiazepines, muscle relaxant, and antidepressants.
Ethical concerns
This study was approved by the ethical committee of the Isfahan University of Medical Sciences (project number 394307). Consent form which included the aim of the study and safety and efficacy of the study was assigned by parents of children.
Treatment options
The eligible children were randomly (simple randomization) allocated to one of the following groups: (1) moderate-dose diazepam: 5 mg for 2–8-year-old children and 10 mg for 9–15-year-old children;[9] (2) low-dose diazepam: 2.5 mg for 2–8-year-old children and 5 mg for 9–15-year-old children;[9] (3) placebo group.
Each participant administered one tablet 30 min before bedtime in the first 5 days and then every other night for the resting part up to 2 weeks. For little children who could not take a pill, the parents were advised to crush it and place it in a small amount of food that the child likes juice, food, etc.
Tablets and placebo were prepared by a drug company (Amin Pharmaceutical Company, Isfahan, Iran) with similar shapes and sizes, in two different colors which were used for two different age groups. Each tablet had special code that was only identified by the company. Researchers, patients, and statistician were blind to the codes.
Outcomes and outcome measurement
The primary outcome was the bruxism severity score (BSS). The severity score consisted of three components: (1) frequency of episodes per week, defined as the number of nights in the prior week in which at least one attack of SB was noticed by parents, (2) frequency of episodes per night, which was the mean number of episodes per night in the prior week, and (3) duration of SB, defined as the approximate total duration of SB during night (in minutes) in the prior week.[10] The parents were asked to sleep near their child during the study for more accurate reporting of SB episodes. Scoring was applied according to Table 1, which was created by the senior researchers of the project and the sum of scores presented the BSS.
Table 1.
Determination of bruxism severity score in study children
| Frequency per week | Score | Frequency per night | Score | Duration per attack | Score |
|---|---|---|---|---|---|
| Less than three nights | 2 | Once | 1 | >15 min | 4 |
| Most nights | 3 | More than once | 2 | 5–15 min | 2 |
| Every night | 4 | - | - | <5 min | 1 |
The sum of scores is the bruxism severity score
The secondary outcome was the unfavorable side effects of the intervention such as next-day drowsiness and vertigo.
The primary outcome was assessed at the beginning of the study (T0) and at three follow-ups on weeks 2, 4, and 12 of the study (T1, T2, and T3, respectively). Side effects were assessed at the end of the second week of the intervention (T1).
At the beginning, parents completed a questionnaire containing medical history, drug history, and demographic information of the children. One expert pediatric resident examined the children, assessed the criteria for inclusion, allocated the patients, and filled out the forms of disease severity and side effects. In addition, she reassessed adherence of the children, severity indices of SB, and adverse effect of medication by calling the parents on weeks 2, 4, and 12 of the study and filling out the forms of the study. The way of taking medication and possible side effects was described both orally and by a written material to the parents. In addition, the phone number of the responsible resident was given to the parents for asking their questions and reporting severe side effects.
Statistical analysis
The data were analyzed using SPSS-20 for Windows (SPSS, Inc., Chicago, IL, USA). A per-protocol strategy was used for statistical analysis. Quantitative normally distributed data are presented as mean ± standard deviation (SD) and categorical data as frequency (percentage). One-way analysis of variance (ANOVA) was used to compare basic characteristics and simple effects between treatment groups. A mixed-model ANOVA was performed with the treatment groups as between-subject variable and time of measurement of BSS as within-subject variable. Simple repeated-measure ANOVA was done to detect the difference between follow-up time points with the baseline BSS in different groups. Post hoc Bonferroni test was applied for multiple pairwise comparisons. Logistic regression analysis was used to compare the rate of occurrence of side effects between treatment groups. P < 0.05 was considered statistically significant.
RESULTS
One hundred and nine patients who referred for bruxism were enrolled in the study. Nineteen children were dropped out during the study because of sleepiness and/or lack of compliance for medication use. Finally, data obtained from 90 children with SB were analyzed. The flow diagram of the study is shown in Figure 1. Mean (SD) age of the participants was 7.37 (3.10) and 48.9% were boys. Distribution of sex, age, and mean pretreatment BSS (T0) of participants are shown in Table 2. Age and sex distribution were not significantly different between groups; however, mean pretreatment BSS was significantly different; thus, this variable was interred in the model as a covariate.
Figure 1.
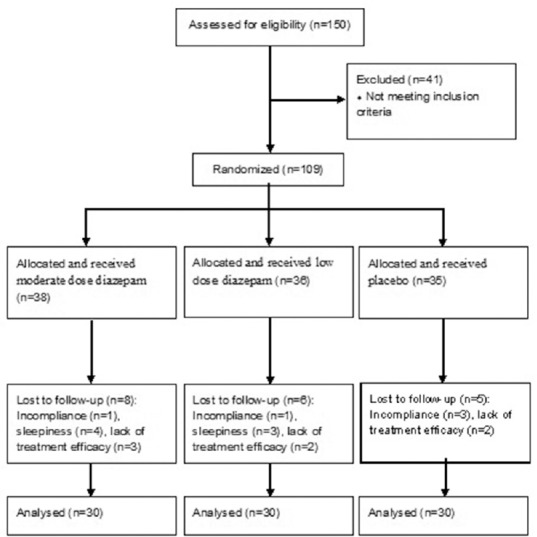
Trial profile of the randomized, placebo-controlled trial of diazepam for bruxism in 2–15-year-old children in Isfahan, Iran
Table 2.
Basic characteristics of children randomized to placebo, low-dose diazepam, and moderate-dose diazepam groups
| Basic characteristics | Placebo | Low dose diazepam | Moderate dose diazepam | P |
|---|---|---|---|---|
| Sex(%) | ||||
| Male | 16 (53.3) | 18 (60.0) | 10 (33.3) | 0.099a |
| Female | 14 (46.7) | 12 (40.0) | 20 (66.7) | |
| Age (mean±SD) | 6.95±2.48 | 7.80±3.80 | 7.35±2.90 | 0.574b |
| Bruxism severity score at pretreatment time (mean±SD) | 6.13±1.46 | 7.13±1.33 | 6.60±1.55 | 0.032b |
aBy Chi-square test, bBy one-way ANOVA. ANOVA=Analysis of variance, SD=Standard deviation
Normality was checked by Shapiro–Wilk test. As the assumption of sphericity was violated in Mauchly's test (χ2 (2) = 50.11, P = 0.0001), Greenhouse-Geisser correction was applied. Handling T0 as a covariate, there was a significant main effect for time (f(1.38, 119) = 4.37, P = 0.027) and time–group interaction (f(2.77, 119) = 3.37, P = 0.024). Pairwise comparison with Bonferroni correction demonstrated significant difference in mean BSS between T1 with T2 and T3 (P = 0.0001 for both comparisons) but not between T2 and T3 (P = 1.00). In other words, BSS increased significantly from T1 to T2 and remained at the same level at T3 [Table 3]. However, group of intervention did not have a main effect on BSS (f(1, 86) =0.61, P = 0.554), i.e., the mean BSS did not differ significantly between groups [Table 3].
Table 3.
The mean severity score of bruxism in children randomized to placebo, low dose and moderate dose diazepam groups before and throughout the study
| Groups | Time of follow up, mean±SD | P value a time effect: 0.027 | P value a time×group: 0.024 | P value a between groups: 0.544 | |||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||
| Placebo | 6.13±1.46 | 5.10±1.47 | 5.97±1.63 | 5.93±1.53 | 0.000 | ||
| Low dose diazepam | 6.60±1.04 | 4.90±1.24 | 6.67±1.47 | 6.33±1.32 | 0.000 | ||
| Moderate dose diazepam | 6.60±1.54 | 4.70±1.12 | 6.20±1.58 | 6.30±1.53 | 0.000 | ||
| Pb | 0.032 | 0.487 | 0.052 | 0.356 | |||
aBy repeated measure ANOVA (T0 as a covariate), bBy one-way ANOVA. T0=Pretreatment, T1=Week 2, T2=Week 8, T3=Week 12, SD=Standard deviation, ANOVA=Analysis of variance
One-way ANOVA was performed separately for each time point in different groups [Table 3]. There was no significant difference in mean BSS between groups at any time point (except for the T0). The statistical analysis did not show any significant effects for low or intermediate dose of diazepam on the severity of SB in the studied groups.
Overall, 10% of placebo group, 33.3% of low-dose group, and 43.3% of moderate-dose group reported next-day drowsiness at week 2 of the intervention. In logistic regression analysis adjusted for age of the participants, the incidence of next-day drowsiness was significantly different between the groups of patients (P = 0.026). The groups using diazepam showed significant increase in this side effect compared with the placebo group (low-dose diazepam vs. placebo, odds ratio [OR]: 4.41, 95% confidence interval [CI]: 1.06–18.24 and moderate-dose diazepam vs. placebo, OR: 6.82, 95% CI: 1.69–27.54). No other adverse event was reported by the parents.
DISCUSSION
In this trial, we evaluated a short course effect of low dose and moderate dose of diazepam on the severity of SB in children. Our results showed no substantial effect of diazepam in reducing the severity of SB in children.
SB is a multifactorial disorder with various adverse consequences including headaches, tooth wear/fracture, implant, temporomandibular disorders, and other restoration failure. Although several studies have evaluated the effectiveness of different treatment modalities in the management of SB, there is no definitive treatment for SB. Reported management strategies for SB are oral appliance treatments, pharmacotherapy, and behavioral strategies for the protection of tooth/restoration and reduction of SB activity and its related pain. Results of recent review studies have indicated that there are no appropriate cures for SB.[11,12]
Results of a Cochrane review study in pharmacotherapy of SB indicated that there is not enough evidence-based, well-designed, RCTs with large sample size studies in this field.[13]
Based on the findings of Yap and Chua, although the use of different pharmacological agents had been reported, there are only controlled clinical trials for effectiveness of clonidine, L-dopa, and clonazepam in the management of SB.[6,14,15,16]
Evidence indicated that from the above-mentioned agents, benzodiazepines could improve subjective sleep and awakening quality due to their hypnotic, anxiolytic, myorelaxant, and anticonvulsive effects; however, some related adverse effects including some psychological side effects and risk of dependency limit long-term use of the agents in this field. Clonazepam was the most frequently used benzodiazepine in this field.[13,14]
There are few studies regarding the effectiveness of diazepam in the treatment of SB. Montgomery et al. reported that short-term use of diazepam reduced bruxism in 11 patients. Furthermore, they found that nocturnal masseter electromyographic activity was significantly reduced following the usage of diazepam at bedtime in patients suffering from clinical symptoms of masticatory hyperactivity.[17,18] Johar reported a case of SB-treated by behavioral change using diazepam for hypnosis.[19] In addition, based on experience, some practitioners used diazepam as an effective treatment for nocturnal bruxism.
Our study was the first study in childhood SB that assessed the real effect of diazepam in a double-blinded trial. We found no significant effect on the drug in the studied population. Hence, we suggest that another medication or other measures such as cognitive behavioral techniques should be investigated in future studies.
Researchers recommend caution when advising benzodiazepines in children due to risk of long-term use in this population and subsequent adverse events such as tolerance, dependency, withdrawal, and cognitive deficits.[7] Hence, we do not recommend using benzodiazepines in children with SB due to lack of significant efficacy and risk of significant long-term adverse effect.
Our findings showed that mean BSS reduced significantly in all groups, i.e., those receiving low-dose, moderate-dose, and placebo groups after 2 weeks of consuming diazepam. This effect was of short duration, and thereafter, the mean scores returned to nearly the pretreatment scores in all groups. This finding elucidates that placebo effect of consuming any medication might transiently reduce SB episodes or SB report by parents in children. Previous studies showed the efficacy of psychosocial interventions such as counseling, hypnosis, relaxation exercises, and biofeedback in the reduction of bruxism frequency and intensity.[20] Our experience in reduction of BSS in placebo group could be attributed to psychological aspects of the disorder. On the other hand, the positive effect in reducing bruxism in nonblind studies or clinical experiences can be endorsed to placebo effect, which could be achieved by consumption of any medication including placebo. Thus, we considered that clinical trials on SB should be blind enough to be able for detecting the real effect of interventions.
We found a high rate of next-day drowsiness in active drug groups compared to the placebo group. This is a predictable consequence of benzodiazepines, which could restrict their use in benign self-limited conditions of children such as SB.[7]
Our study has some limitations. We relied on parental reports without using polysomnographic and/or audio/video home recordings in a sleep laboratory for accurate diagnosis and assessment of SB severity in studied children. Therefore, data obtained from this study are subjective and do not contain SB events which were not accompanied by sound or episodes which occurred when parents were not awake. Further, we evaluated adherence of children, severity of SB episodes, and adverse effects of medication only by phone follow-up, which could not be as accurate as face-to-face assessment. Finally, our groups lacked homogeneity in the basic mean BSS which could interfere with the effect of the intervention although this was considered in the statistical analysis. The strength of the study is the well-designed study in regard to its sample size and blinding and considering placebo group.
CONCLUSION
The results of this study do not support the use of diazepam for the management of SB in otherwise normal children due to lack of substantial benefit and presence of adverse events. It is suggested that using benzodiazepines such as diazepam in combination with other treatment modalities would be more effective for the treatment of SB which should be evaluated in further studies.
Financial support and sponsorship
Isfahan University of Medical Sciences (project number 394307) supported the study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work has been performed in Emam Hossein Children's Hospital, and financially supported by the Vice-Chancellery for Research of Isfahan University of Medical Sciences.
REFERENCES
- 1.Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ, et al. Bruxism defined and graded: An international consensus. J Oral Rehabil. 2013;40:2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 2.Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: A systematic review of the literature. J Oral Rehabil. 2013;40:631–42. doi: 10.1111/joor.12069. [DOI] [PubMed] [Google Scholar]
- 3.Shetty S, Pitti V, Satish Babu CL, Surendra Kumar GP, Deepthi BC. Bruxism: A literature review. J Indian Prosthodont Soc. 2010;10:141–8. doi: 10.1007/s13191-011-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freitas AR, Dias MM, Falcão Filho HB, Vasconcellos AA. Sleep bruxism in children: Prevalence and multidisciplinary therapy. Oral Health DentManage. 2014;13:897–901. [Google Scholar]
- 5.Saletu A, Parapatics S, Saletu B, Anderer P, Prause W, Putz H, et al. On the pharmacotherapy of sleep bruxism: Placebo-controlled polysomnographic and psychometric studies with clonazepam. Neuropsychobiology. 2005;51:214–25. doi: 10.1159/000085917. [DOI] [PubMed] [Google Scholar]
- 6.Saletu A, Parapatics S, Anderer P, Matejka M, Saletu B. Controlled clinical, polysomnographic and psychometric studies on differences between sleep bruxers and controls and acute effects of clonazepam as compared with placebo. Eur Arch Psychiatry Clin Neurosci. 2010;260:163–74. doi: 10.1007/s00406-009-0034-0. [DOI] [PubMed] [Google Scholar]
- 7.Griffin CE, 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Witek MW, Rojas V, Alonso C, Minami H, Silva RR. Review of benzodiazepine use in children and adolescents. Psychiatr Q. 2005;76:283–96. doi: 10.1007/s11126-005-2982-5. [DOI] [PubMed] [Google Scholar]
- 9.Basow DS, editor. Waltham (MA): Lexicomp, UpToDate; 2018. [Last accessed on 2018 Aug 13]. Diazepam: Pediatric Drug Information. Available from: http:// www.uptodate.com . [Google Scholar]
- 10.Chicago, Illinois: American Academy of Sleep Medicine; 2001. American Academy of Sleep Medicine. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. [Google Scholar]
- 11.Carra MC, Huynh N, Fleury B, Lavigne G. Overview on sleep bruxism for sleep medicine clinicians. Sleep Med Clin. 2015;10:375–84, xvi. doi: 10.1016/j.jsmc.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Huynh N, Manzini C, Rompré PH, Lavigne GJ. Weighing the potential effectiveness of various treatments for sleep bruxism. J Can Dent Assoc. 2007;73:727–30. [PubMed] [Google Scholar]
- 13.Macedo CR, Macedo EC, Torloni MR, Silva AB, Prado GF. Pharmacotherapy for sleep bruxism. Cochrane Database Syst Rev. 2014;10:1–51. doi: 10.1002/14651858.CD005578.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap AU, Chua AP. Sleep bruxism: Current knowledge and contemporary management. J Conserv Dent. 2016;19:383–9. doi: 10.4103/0972-0707.190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh N, Lavigne GJ, Lanfranchi PA, Montplaisir JY, de Champlain J. The effect of 2 sympatholytic medications – Propranolol and clonidine – On sleep bruxism: Experimental randomized controlled studies. Sleep. 2006;29:307–16. doi: 10.1093/sleep/29.3.307. [DOI] [PubMed] [Google Scholar]
- 16.Lobbezoo F, Lavigne GJ, Tanguay R, Montplaisir JY. The effect of catecholamine precursor L-dopa on sleep bruxism: A controlled clinical trial. Mov Disord. 1997;12:73–8. doi: 10.1002/mds.870120113. [DOI] [PubMed] [Google Scholar]
- 17.Winocur E, Gavish A, Voikovitch M, Emodi-Perlman A, Eli I. Drugs and bruxism: A critical review. J Orofac Pain. 2003;17:99–111. [PubMed] [Google Scholar]
- 18.Montgomery MT, Nishioka G, Rugh JD, Thrash WJ. Effect of diazepam on nocturnal masticatory muscle activity. J Dent Res. 1986;65:180. [Google Scholar]
- 19.Johar SQ. A case of sleep bruxism treated through behavioural change using hypnosis. Med J DY Patil Univ. 2012;5:154–7. [Google Scholar]
- 20.Carra MC, Huynh N, Lavigne G. Sleep bruxism: A comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]


