Abstract
Leucine-rich repeat G protein-coupled receptors (LGRs) and their endogenous ligands R-spondin1–4 (Rspo) are critical in embryonic development and in maintenance of stem cells. The functions of the Rspo-LGR system in differentiated cells remain uncharacterized. In this study, the expression profiles of LGRs and Rspos were characterized in mature hepatocytes. A liver-specific knockout of LGR4 in mouse was generated and used to study hepatic ischemia/reperfusion-induced injury (HIRI) as well as lipopolysaccharide/D-galactosamine (LPS/D-Gal)-induced liver injury. We have demonstrated that, in adult liver, LGR4 is expressed in hepatocytes and responds to Rspo1 with internalization. Rspo1 is responsive to various nutritional states and to mTOR signaling. Activation of LGR4 by Rspo1 significantly reduced tumor necrosis factor-α (TNFα)-induced cell death, and levels of NF-κB-p65 and caspase-3 in cultured hepatocytes. Knockdown of hepatic LGR4 rendered hepatocytes more vulnerable to TNFα-induced damage in cultured primary cells and in the setting of HIRI and LPS/D-Gal-induced liver injury. Rspo1 potentiated both basal and Wnt3a-induced stabilization of β-catenin. Disruption of β-catenin signaling reversed the protective effects of Rspo1 on TNFα-induced hepatocyte toxicity. LGR4 knockdown increased nuclear translocation of NF-κB-p65 in response to acute injury. Overexpression of IKKβ attenuated the protective effects of Rspo1 on TNFα-induced cell death. In conclusion, the Rspo1-LGR4 system represents a novel pathway for cytoprotection and modulation of stress-induced tissue damage.
NEW & NOTEWORTHY Functional LGR4 is present in mature hepatocytes. R-spodin1 protects hepatocytes from tumor necrosis factor-α-induced cell death. Liver-specific knockdown of LGR4 renders liver more susceptible to acute injury. LGR4 protects hepatocytes from injury by inhibition of NF-κB signaling.
Keywords: apoptosis, HIRI, R-spondin1, Wnt/β-catenin signaling
INTRODUCTION
Leucine-rich repeat G protein-coupled receptors (LGRs), a highly conserved GPCR family, are characterized by a large extracellular domain with multiple copies of leucine-rich repeats (LRR) (12). LGRs 4–6 compose a subgroup of LGRs, each containing 17 LRRs (7). LGRs are important for embryonic development and maintenance of stem cells. LGR4 is widely expressed in proliferating cells in embryos and adults (25). In the digestive system, LGR4 plays key roles in intestinal development and homeostasis. Furthermore, LGR4 is critical for postnatal epithelial cell proliferation and terminal Paneth cell differentiation. LGR4 mutant mice are more susceptible to chemically induced inflammatory bowel disease (17). Recently, a study by Planas-Paz et al. (19) highlighted the RSPO-LGR4/5-ZNRF3/RNF43 module in developing liver stem cells as a master regulator of liver zonation and as a control mechanism for growth/size control during liver development, homeostasis, and regeneration. The roles of LGR4 in differentiated-hepatocyte injury have not been described.
The endogenous ligands for LGRs are four secreted R-spondin proteins (Rspo1-4) that act to potentiate Wnt/β-catenin signaling (24). Rspo proteins share ~40–60% amino acid sequence identity and substantial structural homologies between human and mouse (6). Rspo proteins act as potentiators for Wnt signaling and exhibit ligand properties. The Wnt signaling pathway plays pivotal roles in diverse biological processes during embryonic development, adult homeostasis, and disease pathogenesis (15, 22). Rspo proteins may contribute to these biological processes. Indeed, Rspo1 inhibits osteoclast development and bone resorption, thus preventing bone erosion and improving cartilage integrity in a tumor necrosis factor-α (TNFα)-overexpressing arthritis mouse model (8). In acute and chronic models of colitis in mice, administration of Rspo1 preserves mucosal integrity in both small and large bowel by stimulating crypt epithelial cell mitosis (26). Combined with Slit2, Rspo1 protects intestinal stem cells from chemoradiotherapy-induced damage (27). These findings indicate that the Rspo-LGR4 system is critical for stem cell integrity. Whether this system affects functional homeostasis of differentiated mature cells is unknown. In the present study, we report the existence of Rspo1-LGR4 signaling in differentiated hepatocytes of adult liver and the ability of Rspo1-LGR4 to protect hepatocytes against acute injury.
MATERIALS AND METHODS
Materials
Mouse anti-LGR4, TNFα, and Rspo1 were purchased from R&D Systems (Minneapolis, MN). Lipopolysaccharide (LPS) and D-galactosamine (D-Gal) were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit anti-NF-κB-p65 and rabbit anti-caspase-3 polyclonal antibody were obtained from Abcam (Cambridge, MA). Rabbit anti-GAPDH and lamin B were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-S6 (Ser235/236) and S6 rabbit monoclonal antibodies, rabbit anti-phospho-NF-κB-p65, mechanistic target of rapamycin (mTOR) rabbit monoclonal antibodies, and rabbit anti-β-actin antibody were purchased from Cell Signaling Technology (Beverly, MA). TRIzol reagent and the reverse transcription (RT) system were from Invitrogen (Carlsbad, CA). The cell death ELISA kit (cat. no. 11 544675001) and in situ cell death detection POD kit (cat. no. 11684817910) were from Roche (Penzberg, Germany). The live green caspase-3 and -7 detection kit was from Molecular Probes, Invitrogen Detection Technologies (Eugene, OR).
Animals and Treatments
Animals.
Animals were housed in a temperature-controlled environment with 12-h:12-h light-dark cycles, and access to food and water ad libitum. All experimental protocols were approved by University Committee on the Use and Care of Animals of the University of Michigan. Twelve-week-old male mice were used in the present study. Albumin-Cre mice (C57BL/6J background) were purchased from Jackson Laboratory (Bar Harbor, ME). Four mouse embryonic stem (ES) cell clones (from C57BL/6J, black, XY genotype) with FRT-flanked LacZ and neo cassettes prior floxed exons 15 and 16 of the LGR4 gene were purchased from EUCOMM, Helmholtz Zentrum (Munich, Germany). Knock-in mice were created at the University of Michigan Transgenic Core. LGR4floxp mice were generated by mating the male heterozygous LGR4floxp mice with B6 mice. 129S4-Gt(ROSA)26Sortm2(FLP*)Sor/J mice were purchased from Jackson Laboratory to delete the Lacz gene and then mated with wild-type (WT) C57BL/6J mice to generate LGR4floxp transgenic mice free of Laz and Flp genes.
Generation of Alb-LGR4−/− mice.
Alb-LGR4−/− (AL) mice were generated by cross-breeding Albumin-Cre mice with LGR4floxp/floxp mice. Deletion of hepatic LGR4 was confirmed by genotyping and immunofluorescence.
Hepatic ischemia/reperfusion-induced injury.
Twelve-week-old WT or genetically engineered male mice were anesthetized with ketamine (100 mg/kg bodywt) and xylazine (6 mg/kg bodywt) by intraperitoneal injection. The liver was exposed by midline laparotomy, and the hepatic artery and the portal vein were clamped using an atraumatic vascular clip. This procedure has been reported to cause segmental hepatic ischemia and to prevent mesenteric venous congestion by allowing portal decompression throughout the right and caudate lobes of the liver. Blood flow was interrupted for 75 min to induce hepatic ischemia, and reflow was initiated by removal of the vascular clip to allow reperfusion for 6 h. At the end of the reperfusion period, plasma and representative samples of the ischemic and unaffected hepatic lobes were collected and stored at −80°C.
Acute liver injury induced by LPS/D-Gal injection.
Twelve-week-old AL mice and WT littermates were intraperitoneally injected with lipopolysaccharide (LPS; 20 μg/kg) and D-galactosamine (D-Gal; 400 mg/kg) dissolved in PBS to induce acute liver injury or with PBS in the control mice. Mice were euthanized at 6 h after LPS/D-Gal injection.
Tail vein injection of adenovirus.
Twelve-week-old LGR4flox/flox mice and WT littermates were administrated Cre adenovirus (Ad-Cre) (109 pfu/mouse) via tail vein injection. Twelve-week-old C57BL/6J mice were injected with the Ad-IKKβ adenovirus (109 pfu) through the tail vein for 1 wk. GFP adenovirus (Ad-GFP) was used as control.
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
A TUNEL assay was performed according to the manufacturer’s instructions. Frozen hepatic slides were fixed with 4% paraformaldehyde, immersed in 3% H2O2 in methanol to block endogenous peroxidase (POD) and then permeabilized with 20 μg/ml proteinase K. TUNEL reaction was performed for 1 h with labeling mix in a 37°C incubator, and the fluorescent signal was observed. Alternatively, the reaction was converted to a POD-labeled signal and visualized with DAB substrate. Stained cells were analyzed under fluorescent or light microscope.
Primary Hepatocyte Isolation and Culture
C57BL/6J mice were anesthetized as described above and injected (ip) with 1,000 IU of heparin. After laparotomy, the portal vein was cannulated. The liver was perfused with 20 ml of prewarmed 37°C Hanks buffer, followed by 20 ml of 0.02% collagenase (Sigma-Aldrich, Burlington, MA) at a flow rate of 2 ml/min. After perfusion, liver tissues were removed and washed with 20 ml of Hanks buffer. The capsule of the liver was removed, and hepatic tissues were dispersed and incubated in 20 ml of 0.01% collagenase in a shaking water bath at 37°C for 15 min. Cell suspension was then filtered through two layers of 60- to 80-μm nylon mesh, centrifuged at 500 rpm, and washed twice with DMEM to remove tissue dissociation enzymes, damaged cells, and nonparenchymal cells. Dispersed hepatocytes were counted and seeded at a concentration of 1 × 105 cells per well in a six-well plate containing 2 ml of high-glucose DMEM supplemented with 10% (vol/vol) FBS. Cells were cultured at 37°C in a humidified atmosphere of 5% (vol/vol) CO2.
Immunofluorescence Staining
Primary hepatocytes from WT animals or liver tissue slides were washed with PBS, fixed with 4% paraformaldehyde, and then immersed in 0.02% Triton X-100 in PBS for permeabilization. Goat serum (5%) was used for blocking. Primary antibody was incubated with cells or tissues overnight in a 4°C refrigerator. Secondary antibody was incubated in room temperature for 2 h. Nuclei were stained with DAPI.
Western Blot Analysis
Liver tissue and cultured cells were harvested and homogenized in lysis buffer with proteinase inhibitor cocktail (Sigma-Aldrich, Burlington, MA). Lysates were then centrifuged at 20,000 rcf for 15 min at 4°C, and supernatants were transferred to prechilled fresh tubes. Protein concentration in cell lysates was determined using the BCA protein assay (Thermo Scientific, Waltham, MA). Samples were boiled for 5 min, cooled on ice for 1 min, and vortexed, and equal protein amounts were separated on gradient polyacrylamide gels (Invitrogen) and then transferred to a polyvinylidene fluoride membrane. Membranes were incubated with 5% fat-free milk in Tris-buffered saline containing Tween 20 for 1 h at room temperature, followed by incubation with primary antibodies overnight at 4°C. Specific reaction was detected using IRDye-conjugated secondary antibody and visualized using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Quantitative PCR
For gene expression analysis, RNA was isolated from mouse liver or hepatic primary cells by TRIzol (Invitrogen) and reverse-transcribed into cDNAs using the First-Strand Synthesis System for RT-PCR kit (Invitrogen). SYBR Green-based quantitative RT-PCR was performed using the Mx3000 multiplex quantitative PCR system (Stratagene). Triplicated samples were used for each experimental condition to determine relative expression levels.
Statistics
All values are expressed as means ± SE. Statistical differences were evaluated by two-way ANOVA and Newman-Student-Keuls test. Comparisons between two groups involved the use of the Student’s t-test. A P value of <0.05 denotes statistical significance.
RESULTS
Existence of Functional LGR4 in Hepatocytes
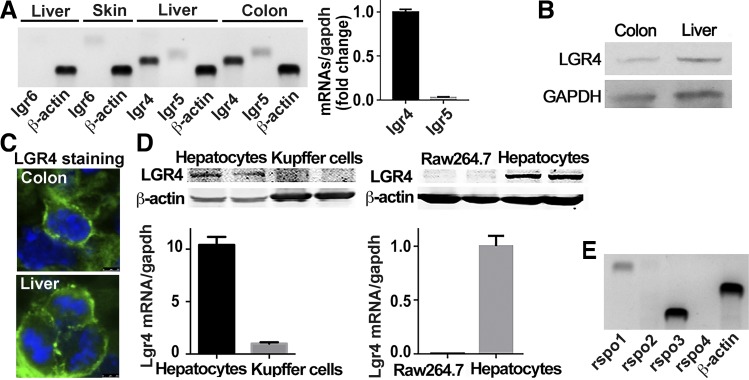
Using skin and colon tissues as positive control for LGR6 (21) and LGRs 4/5 (14) respectively, we detected the expression levels of LGRs 4–6 in liver. LGR6 mRNA was detectable in skin but not in the liver tissue. Both LGRs 4 and 5 were visible in liver and colon; the expression levels of LGR4 were much higher than LGR5 in the liver (Fig. 1A). Consistently, LGR4 protein was detected in both colon and liver (Fig. 1B). Immunostaining for LGR4 showed that it was expressed in the membrane of typical binucleate hepatocytes (Fig. 1C). Furthermore, LGR4 mRNA and protein were abundant in primary hepatocytes rather than in isolated Kupffer cells and Raw 264.7 cells, a mouse monocyte/macrophage cell line (Fig. 1D). Since primary hepatocytes were usually dediffereniated after 48 h in in vitro culture, we detected the expression levels of LGR4 at various time points during in vitro culture. LGR4 mRNA levels remained steady for up to 24 h and then increased from 36 to 72 h in culture (data not shown). Therefore, in vitro experiments using primary hepatocytes were performed within 24 h except for those with adenovirus infection, which require a total of 36 h for transfection and treatment. Moreover, Rspo1 and Rspo3, the endogenous ligands for LGRs, were expressed in liver tissue, whereas Rspo2 and Rspo4 were not detectable (Fig. 1E).
Fig. 1.
Existence of leucine-rich repeat G protein-coupled receptors (LGRs) in hepatocytes. A: LGR expression in liver. RT-PCR was performed to detect mRNA expression of lgr4/5/6 in liver. Skin was used as a positive control for lgr6, and colon was used as positive control for lgr4 and lgr5; β-actin was used as loading control of PCR. B: LGR4 protein in liver. Western blot was performed to detect the expression of LGR4 protein in colon and liver. GAPDH was used as control. C: LGR4 expression in hepatocytic membrane. Immunofluorescent staining was used to detect the cellular expression of LGR4 in colon and liver. Green indicates LGR4 protein; blue indicates nuclei. D: differential expression of LGR4 in macrophages and hepatocytes. Primary hepatocytes and Kupffer cells were isolated from C57BL/6J mice. RNA and protein were extracted from primary hepatocytes, Kupffer cells, and cultured Raw 264.7 cells and analyzed by quantitative RT-PCR and Western blot; n = 6. E: expression of R-spondins (rspos) in liver. Liver tissues were obtained from C57/BL6J mice. Hepatic RNAs were extracted and analyzed by quantitative RT-PCR; β-actin was used as loading control; n = 6.
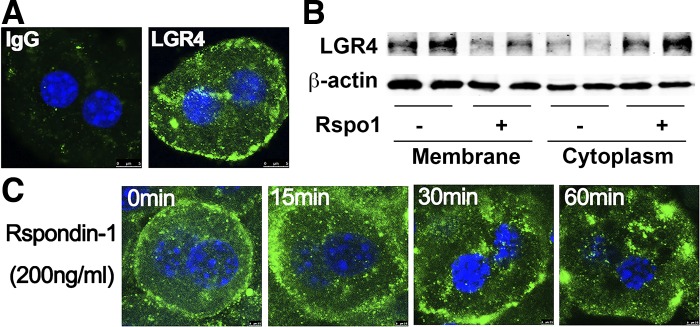
Primary hepatocytes were stained for LGR4 by use of a specific antibody, and LGR4 immunoreactivity was localized to the cellular membrane (Fig. 2A). To determine whether the LGR4 receptor in hepatocytes is functional, cells were treated with Rspo1 (200 ng/ml) for 60 min, and membrane and cytoplasm proteins were extracted and detected for LGR4. We observed the LGR4 protein translocated from membrane to cytoplasm upon Rspo1 treatment (Fig. 2B). Moreover, hepatocytes were incubated with Rspo1 for various times at 15, 30, and 60 min and then stained with LGR4 antibody. Time-dependent internalization of LGR4 from cellular membrane to cytosol, typical of G protein coupled receptors, was demonstrated (Fig. 2C).
Fig. 2.
Functional leucine-rich repeat G protein-coupled receptors (LGRs) in hepatocytes. A: LGR4 immunoreactivity in hepatocytes. Isolated hepatocytes were stained with LGR4 antibody (green), and nuclei were stained with DAPI (blue). B: translocation of LGR4 upon treatment with R-spondin-1 (Rspo1). Isolated hepatocytes were incubated with Rspo1 (200 ng/ml) or saline for 60 min, and membrane and cytoplasm proteins were isolated and used for Western blot to detect LGR4 translocation; β-actin was used as loading control. C: internalization of LGR4. Isolated hepatocytes were incubated with Rspo1 (200 ng/ml) for the time indicated and then stained with LGR4 antibody (green) to detect mobilization of LGR4 receptor upon activation by Rspo1. Each experiment was repeated 3 times.
Alteration of Rspo1, an Endogenous Ligand of LGR4, in Response to Nutritional Status
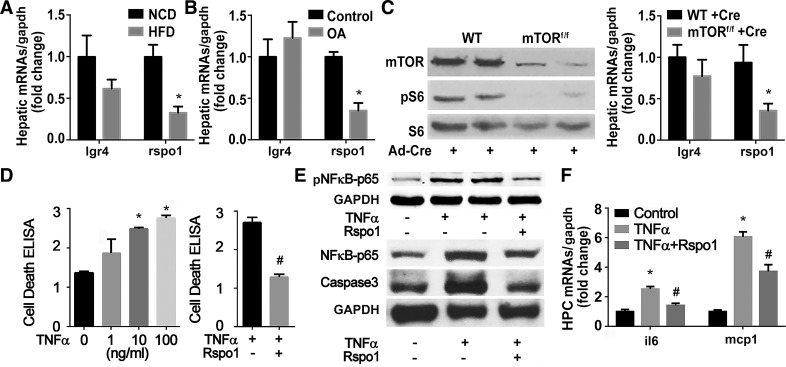
We next examined the expression of LGR4 and its ligand, Rspo1, in a high-fat diet (HFD)-induced obesity animal model (Fig. 3A) and primary cultured hepatocytes treated with oleic acid (OA) (Fig. 3B). Rspo1 was significantly decreased in HFD-induced steatotic liver and in hepatocytes treated with OA. Since mTOR signaling is critical for hepatic lipid metabolism and injury induced by ischemia/reperfusion (13), the effects of mTOR signaling on the expression of LGR4 and Rspo1 were next measured. Hepatic mTOR signaling was suppressed by deletion of mTOR gene through injection of adenovirus-Cre (Ad-Cre) into mTORflox/flox mice. Suppression of hepatic mTOR signaling, as evidenced by a decrease in the phosphorylation of S6, a downstream target of mTOR, significantly inhibited the expression of Rspo1 but not of LGR4 (Fig. 3C). Since deletion of mTOR in liver facilitates hepatocyte injury (13), we hypothesized that Rspo1 might play a role in the cytoprotection of hepatocytes. The direct effects of Rspo1 on hepatocyte protection were first examined in cultured primary hepatocytes. TNFα induced dose-dependent increases in cell death. Rspo1 dose-dependently reduced levels of cell death, with 200 mg/ml as the most effective dosage (data not shown), which completely abolished the TNFα-induced cell death (Fig. 3D). Furthermore, Rspo1 treatment diminished the TNFα-induced high levels of phospho-NF-κB-p65, NF-κB-p65, and caspase-3, as well as inflammatory factors including interleukin 6 (il6) and monocyte chemoattractant protein-1 (mcp1) (Fig. 3E).
Fig. 3.
Modulation and protective function of R-spondin-1 (Rspo1) in hepatocytes. A and B: regulation of Rspo1 by energy surplus. Rspo1 mRNA was detected in liver tissue from mice fed normal chow diet (NCD) or high fat diet (HFD) for 12 wk or in hepatocytes cultured with oleic acid (OA; 62.5 μM) for 24 h. *P < 0.05 vs. NCD or control. Each experiment was repeated 3 times. C: regulation of Rspo1 by suppression of hepatic mechanistic target of rapamycin (mTOR). mTOR was knocked down in 12-wk-old mTORflox/flox mice via tail vein injection of Ad-Cre (109 pfu/mouse). Wild-type littermates (WT) were used as controls. One week later, mice were euthanized, and liver proteins and RNAs were extracted and analyzed by Western blot and RT-PCR. Western blot confirmed the deletion of mTOR and suppression of mTOR signaling; n = 6. *P < 0.05 vs. WT treated with Ad-Cre. D–F: protection of hepatocytes against tumor necrosis fctor-α (TNFα)-induced injury by R-spondin1(Rspo1). Primary hepatocytes were isolated from C57BL/6J mice and treated with different doses of TNFα to induce cell death. Rspo1 (200 ng/ml) was added to hepatocytes pretreated with TNFα (100 ng/ml). Cell death (D), NF-κB-p65, cleaved caspase-3 (E), and inflammatory factors (F) were examined by ELISA, Western blot, and RT-PCR 12 h later. *P < 0.05 vs. control without TNFα treatment; #P < 0.05 vs. TNFα-treated group.
LGR4 Knockdown Renders Hepatocytes Susceptible to TNFα-Induced Injury
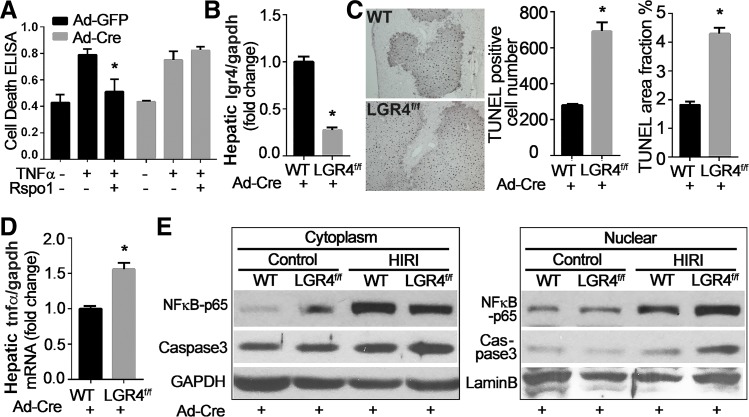
We then evaluated the effects of LGR4 knockdown on the susceptibility of cultured hepatocytes to TNFα-induced injury. Hepatocytes isolated from LGR4flox/flox mice were treated with Ad-Cre to knock down LGR4. Ad-GFP was used as control. Knockdown of LGR4 abolished the protective effect of Rspo1 on TNFα-induced cell death (Fig. 4A). In vivo studies used HIRI as a model (1). HIRI is an established and well-studied experimental paradigm, with quantifiable injury patterns. Knockdown of hepatic LGR4 by tail vein injection of Ad-Cre to LGR4flox/flox mice (Fig. 4B) for 1 wk rendered the liver more vulnerable to HIRI as measured by TUNEL assay (Fig. 4C), and expression levels of TNFα (Fig. 4D), NF-κB-p65, and caspase-3 (Fig. 4E). Translocation of cytoplasmic NF-κB-p65 into nuclei and levels of hepatic caspase-3 were significantly increased in LGR4flox/flox mice treated with Ad-Cre relative to WT littermates.
Fig. 4.
Knockdown of leucine-rich repeat G protein-coupled receptor 4 (LGR4) by adenovirus renders hepatocytes more vulnerable to injury. A: LGR4flox/flox hepatocytes treated with Cre adenovirus (Ad-Cre) are more vulnerable to tumor necrosis factor-α (TNFα)-induced injury. Hepatocytes isolated from LGR4flox/flox mice were treated with adenovirus with green fluorescent protein (Ad-GFP) or Cre (Ad-Cre) for 24 h and then exposed to TNFα (100 ng/ml) with or without R-spondin1 (Rspo1, 200ng/ml) for an additional 12 h. Cell damage was measured by ELISA. *P < 0.05 vs. TNFα. B–E: LGR4flox/flox mice treated with Ad-Cre are more vulnerable to hepatic ischemia/reperfusion injury. Twelve-week-old LGR4flox/flox mice and wild-type littermates (WT) were administered with Ad-Cre (109 pfu/mouse) via tail vein injection. One week later, mice were challenged with hepatic ischemia/reperfusion injury (HIRI). Hepatic mRNA levels of LGR4 validated knockdown of LGR4 in liver (B). Liver injury was evaluated by in situ cell death assay (TUNEL; C). TUNEL-positive cell numbers and staining area were calculated with ImageJ software and expressed as means ± SE; n = 8 for each group. *P < 0.05 vs. WT + Ad-Cre. D and E: LGR4 knockout renders NF-κB pathway highly activated. Hepatic RNAs were extracted and used for quantitative RT-PCR to detect the expression of TNFα (D). Liver proteins were separated into cytoplasmic and nuclear fractions and analyzed by Western blot analysis. GAPDH and lamin B were used as internal control for cytoplasmic and nuclear proteins, respectively (E). *P < 0.05 vs. WT + Ad-Cre.
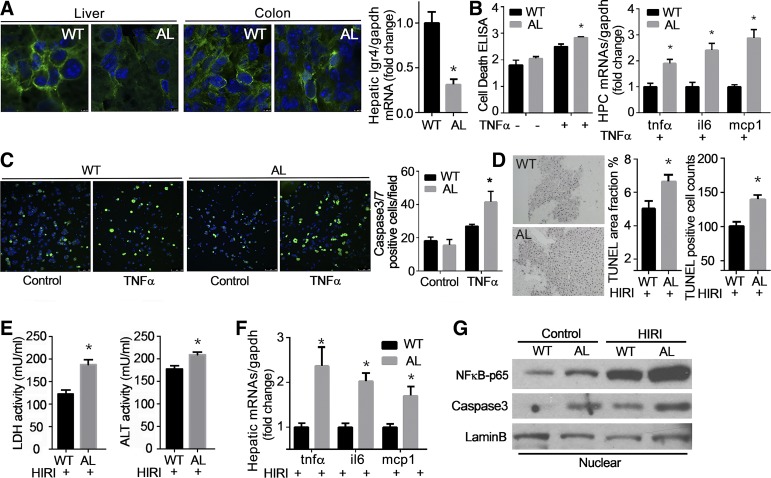
Moreover, liver-specific LGR4-deficient mice (Alb-LGR4−/−) were established by cross-breeding LGR4flox/flox mice with Albumin-Cre mice, and validated by immunofluorescent staining and qPCR showing that LGR4 protein and mRNA levels in liver tissues were lower in Alb-LGR4−/− mice compared with WT littermates (Fig. 5A). Hepatocytes isolated from Alb-LGR4−/− (AL) mice were more vulnerable to TNFα-induced injury, as characterized by increase of cell death and inflammatory factors such as tnfα, il6, and mcp1 (Fig. 5B). Furthermore, TNFα-induced capsase-3 and -7-positive cell numbers detected by live green assay (Fig. 5B) were significantly higher in hepatocytes isolated from Alb-LGR4−/− (AL) mice than WT littermates (Fig. 5C). The in vivo hepatocellular protection of LGR4 was further examined using Alb-LGR4−/− (AL) mice. Liver-specific knockdown of LGR4 was validated by genotyping and immunofluorescent staining. Immunoreactivity and/or mRNA for LGR4 were detected in the colons of Alb-LGR4−/− (AL) and WT mice, as well as hepatic tissue of WT animals, whereas hepatic tissues of Alb-LGR4−/− (AL) mice demonstrated significantly decreased expression (Fig. 5A). Deletion of LGR4 in hepatocytes renders liver more vulnerable to injury as measured by TUNEL assay (Fig. 5D), lactate dehydrogenase/ alanine aminotransferase (LDH/ALT) enzyme activity (Fig. 5E), inflammatory factors (Fig. 5F), and nuclear NF-κB-p65 and caspase-3 levels (Fig. 5G). Animals with hepatic LGR4 knockdown showed no difference in body weight, liver, and adipose tissue weight or in glucose tolerance and insulin sensitivity relative to WT littermates (data not shown). Histological analysis demonstrated normal liver morphology and greater lipid deposition in Alb-LGR4−/− mice. Lipid accumulation in hepatocytes may be attributed to the decrease of lipid utilization consequent to reduction in expression of genes related to β-oxidation (data not shown). Lipid deposition in liver may also play a role in HIRI-induced liver injury, a concept supported by our previous work demonstrating that steatotic liver is more vulnerable to HIRI-induced injury (13).
Fig. 5.
Liver-specific knockdown of leucine-rich repeat G protein-coupled receptor 4 (LGR4) renders hepatocytes more vulnerable to injury. A: liver-specific LGR4 knockdown was confirmed by immunofluorescent staining and quantitative RT-PCR in Alb-LGR4−/− (AL) mice. Liver and colon tissue sections from AL mice and wild type littermates (WT) were stained with LGR4 antibody (green) and DAPI (blue). Quantitative RT-PCR was performed to detect LGR4 mRNA expression. *P < 0.05 vs. WT. B and C: hepatocytes isolated from AL mice are more susceptible to tumor necrosis fctor-α (TNFα)-induced injury. Hepatocytes from AL and WT littermates were treated with TNFα (100 ng/ml) for 12 h. Hepatocellular injury was analyzed by cell death (B), inflammatory factors (B), and caspase-3/7 live green assay (C). *P < 0.05 vs. WT+TNFα. Each experiment was repeated 3 times. D–F: AL mice are more susceptible to hepatic ischemia/reperfusion injury (HIRI). Twelve-week-old mice were challenged with HIRI. Liver injury was evaluated by in situ cell death assay (TUNEL; D). Serum lacate dehydrogenase (LDH) and alanine aminotransferase (ALT) enzyme activities were detected (E). Inflammatory factors such as tnfα, il6, and monocyte chemoattractant protein-1 (mcp1) were detected by quantitative RT-PCR (F). Nuclear fraction proteins were extracted from liver and used for immunoblotting of NF-κB-p65 and caspase-3 (G). *P < 0.05 vs. WT with HIRI; n = 10 for each group.
Deficiency of LGR4 Renders Liver More Vulnerable to LPS/D-Gal-Induced Injury
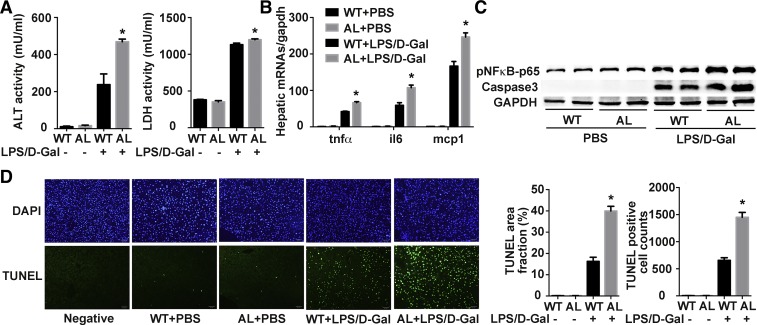
To further validate the protective function of LGR4 in liver, we used another liver injury model, in which damage was induced by LPS and D-Gal coinjection. Consistent with observations in the HIRI animal model, LPS/D-Gal injection induced higher circulating levels of ALT and LDH in Alb-LGR4−/− (AL) mice than in WT littermates (Fig. 6A). Measurement of hepatic inflammatory factors by qPCR also showed that LPS/D-Gal injection significantly increased expression of tnfα, il6, and mcp1 in Alb-LGR4−/− (AL) mice relative to their WT littermates (Fig. 6B). Furthermore, protein levels of phospho-NF-κB-p65 and cleaved caspase-3 were much higher in Alb-LGR4−/− (AL) mice as well (Fig. 6C). Consistent with this observation, TUNEL staining also demonstrated more cell apoptosis in Alb-LGR4−/− (AL) mice than in WT littermates (Fig. 6D). Overall, these studies demonstrate vulnerability to liver injury in animals deficient in LGR4.
Fig. 6.
Deficiency of leucine-rich repeat G protein-coupled receptor 4 (LGR4) renders liver more vulnerable to lipopolysaccharide/D-galactosamine (LPS/D-Gal)-induced injury. Twelve-week-old Alb-LGR4−/− (AL) mice and wild-type (WT) littermates were injected with LPS (20 μg/kg ip) and D-Gal (400 mg/kg ip) dissolved in PBS to induce acute liver injury or with PBS in the control mice. Mice were euthanized 6 h after LPS/D-Gal injection. A: enzyme activities. Serum from WT and AL mice with PBS or LPS/D-Gal injection was used for detection of alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) enzyme activity. *P < 0.05 vs. WT+LPS/G-Gal. B: hepatic inflammatory factors. Hepatic mRNAs from animals were used for qPCR to detect expression levels of inflammatory factors. Gapdh was used as reference gene. *P < 0.05 vs. WT+LPS/G-Gal. C and D: activation of NF-κB-p65 signaling and cell death. Hepatic protein was extracted and used for Western blot to detect phospho-NF-κB-p65 and cleaved caspase-3 levels (C). TUNEL assay was performed to assay for cell death number in liver tissues (D). Green indicates TUNEL-positive cells; blue indicates nuclei; n = 6 per group. *P < 0.05 vs. WT+LPS/G-Gal.
Rspo1 Protects Hepatocytes from Injury through β-catenin/NF-κB Signaling
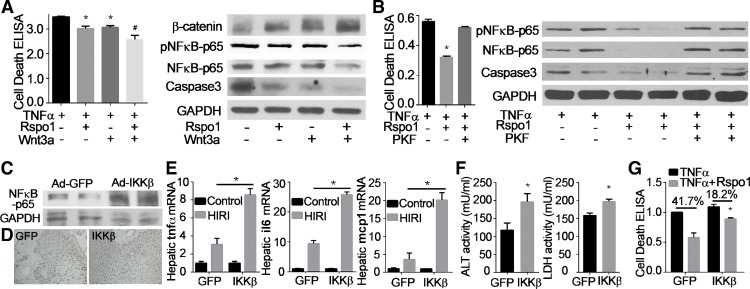
To further investigate the intracellular signaling pathway mediating the effects of LGR4 receptor on hepatic cell death, we studied classical Wnt/β-catenin signaling. We hypothesized that the Rspo-LGR4 system could potentiate Wnt/β-catenin signaling (2). Treatment of cultured hepatocytes with Rspo1 (200 ng/ml) significantly increased levels of β-catenin, as did exposure to Wnt3a (20 ng/ml) (Fig. 7A). Rspo1 potentiated the increases in β-catenin induced by Wnt3a (Fig. 7A). Rspo1-stimulated increases in β-catenin were associated with a significant reduction in TNFα-induced damage, as measured by cell death and expression of NF-κB-p65 and caspase-3 (Fig. 7A). The combination of Rspo1 and Wnt3a significantly increased protection against TNFα-induced injury (Fig. 7A). These observations suggest that Rspo1-LGR4 signaling may affect hepatic cell damage through a Wnt/β-catenin mechanism. This potential mechanism was supported by experiments using T-cell factor (TCF)/β-catenin antagonist PKF115-584, an inhibitor of β-catenin signaling (3). Inhibition of β-catenin signaling by PKF115-584 reversed the protective effect of Rspo1 on TNFα-induced hepatocyte toxicity (Fig. 7B). Changes in cell death, NF-κB-p65, and caspase-3 upon treatment with Rspo1 were reversed by PKF115-584 (Fig. 7B).
Fig. 7.
β-Catenin/NF-κB-dependent mechanism for the cytoprotection of R-spondin1 (Rspo1). A: Rspo1 potentiates cytoprotective effects of Wnt3a. Isolated hepatocytes were treated with tumor necrosis fctor-α (TNFα, 100 ng/ml), Rspo1 (200 ng/ml), and Wnt3a (20 ng/ml) as indicated for 8 h. Cell lysates were used for cell death ELISA and Western blot analysis. B: inhibition of β-catenin attenuates the cytoprotection of Rspo1. Hepatocytes were treated with TNFα (100 ng/ml), Rspo1 (200 ng/ml), and β-catenin signaling inhibitor PKF 115-584 (1 μM) as indicated for 12 h. Hepatocyte damage was examined by cell death ELISA and Western blot analysis for NF-κB and caspase-3. *P < 0.05 vs. vehicle or TNFα. Each experiment was repeated 3 times. C–F: Overexpression of IKKβ increases hepatic ischemia/reperfusion injury (HIRI). IKKβ adenovirus (Ad-IKKβ) (109 pfu/mouse) was administrated via tail vein injection into C57BL/6J mice. Green fluorescent protein adenovirus (Ad-GFP) was used as control. One week later, animals were challenged with ischemia/reperfusion. Activation of NF-κB signaling was validated by Western blot (C). Liver injury was evaluated by in situ cell death assay (TUNEL; D), mRNA levels of inflammatory factors (E) as well as serum alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) enzyme activity (F). *P < 0.05 vs. Ad-GFP with HIRI. G: overexpression of IKKβ attenuates the cytoprotection of Rspo1 against TNFα-induced injury. Hepatocytes from wild type mice were treated with Ad-GFP/IKKβ (5×106 pfu/ml) for 24 h and then exposed to TNFα (100 ng/ml) with or without Rspo1 (200 ng/ml) for 12 h. Cell damage was assessed by ELISA.
β-Catenin forms a complex with the p65 subunit of NF-κB in hepatocytes to inhibit this transcriptional factor (18). Rspo1 potentiated the inhibition of TNFα-induced elevation of NF-κB-p65 by Wnt3a (Fig. 7A). LGR4 knockdown increased nuclear translocation of NF-κB-p65 in response to HIRI (Figs. 4E and 5G). The role of NF-κB in liver injury and hepatocyte survival is pleiotropic, because its activation can be either antiapoptotic or proinflammatory (16). Our studies demonstrate that activation of NF-κB by tail vein injection of IKKβ adenovirus (Fig. 7C) rendered the liver more vulnerable to HIRI, as measured by increase in TUNEL-positive cells, tnfα, il6, and mcp1 (Fig. 7, D and E) and serum levels of ALT and LDH (Fig. 7F). Furthermore, overexpression of IKKβ significantly attenuated the protection of Rspo1 against TNFα-induced cell death in cultured hepatocytes (Fig. 7G). These results indicate a Wnt/β-catenin/NF-κB-dependent mechanism for the Rspo1-LGR4 system in hepatocellular protection.
DISCUSSION
LGR4 is widely expressed in various tissues during embryonic development (25). LGR4 is also distributed in a series of organs in adults such as cartilage, kidney, adrenal gland, testis, nervous system, and digestive system. Our studies demonstrate for the first time the existence of functional LGR4 receptor in differentiated hepatocytes of adult liver. As typical of a membrane receptor, LGR4 undergoes translocation from cellular membrane to cytosol upon binding with Rspo1. Levels of other members of the LGR family, LGR5 and LGR6, are either extremely low or negligible in adult liver. This observation is consistent with a previous report demonstrating the existence of LGR5 near bile ducts in damaged liver (4) but not in healthy adult liver. Rspo-LGR4/5 signaling is also present in liver stem cells. This signaling pathway is critical for liver zonation and regeneration (19).
Using primary hepatocytes and transgenic mice with liver-specific knockdown of LGR4, we observed hepatocellular protection by the Rspo1/LGR4 system during acute injury. This conclusion is supported by the following observations: 1) Rspo1 protected hepatocytes from TNFα-induced cellular injury through β-catenin signaling; hepatocytes with LGR4 knockdown were more susceptible to TNFα-induced cell injury and inflammation. 2) Transgenic animals with liver-specific LGR4 knockdown were more vulnerable to HIRI and injury caused by LPS/D-Gal injection. Although LGR4 is crucial for development, liver-specific knockdown of LGR4 animals exhibited normal body weight, food intake, glucose metabolism, and liver weight, with increased hepatic lipid deposition. Thus, at least part of increased injury evident in LGR4−/− after HIRI or LPS/D-Gal could have been due to the presence of steatosis in these mice, as demonstrated by our previous studies (13). Previous studies have also demonstrated the protective effects of the Rspo1/LGR4 system on intestinal or colonic injury (14, 26). More severe colitis developed in LGR4 mutant mice than in WT littermates when mice were subjected to dextran sulfate sodium (DSS)-induced inflammatory bowel disease. Inflammatory cytokines such as TNFα, IL-6, and IL-1 were significantly increased in LGR4 mutant mice after DSS administration. Stimulation of Wnt/β-catenin signaling with Rspo1 ameliorated 5-fluorouracil and radiation-induced gut damage (27). Although Rspo3 is detected in the liver (unpublished data and Ref. 20), it demonstrates no protective effect on TNFα-induced damage of hepatocytes (data not shown).
Rspo1 is a strong potentiator of Wnt/β-catenin signaling, and β-catenin signaling protects animals from HIRI. More severe hepatic damage has been observed in the β-catenin knockdown mouse in response to ischemia/reperfusion (5, 11). Wnt1-mediated β-catenin stabilization has been reported to increase hepatocyte survival and hepatic resistance to oxidative injury (11). Wnt agonists blunt HIRI-induced elevations of apoptosis and inflammatory cascades and increase hepatic cell proliferation and survival after ischemia/reperfusion (9). Consistent with the protective effects of Wnt/β-catenin signaling, our results demonstrate that Rspo1 potentiates the inhibitory effects of wnt3a on TNFα-induced hepatocellular injury. Inversely, knockdown of Rspo1 receptor-LGR4 rendered the liver more vulnerable to HIRI, and LPS/D-Gal induced liver injury measured by TUNEL assay and inflammatory factor levels. β-Catenin forms a complex with the p65 subunit of NF-κB in hepatocytes to inhibit this transcriptional factor (18). Our studies demonstrated that Rspo1 potentiated the inhibitory effect of Wnt3a on TNFα-induced elevation of NF-κB-p65. LGR4 knockdown facilitated the nuclear translocation of NF-κB-p65. LGR4flox/flox mice with Ad-Cre tail vein injection to knock down hepatic LGR4 expression were challenged with HIRI and showed much higher NF-κB-p65 protein levels in nuclear fractions compared with WT littermates.
However, liver-specific deletion of β-catenin has demonstrated an opposing effect of β-catenin/NF-κB in liver injury (18). Absence of β-catenin in liver renders mice resistant to liver injury, leading to lower morbidity. The following reasons may account for the discrepancy: Rspo1/LGR4 may potentiate the activation of Wnt/β‐catenin signaling through LRP5/6 (12). In our liver-specific LGR4 knockout animal, there is still basal activation of β-catenin, as well as the interaction with NF-κB-p65. In the β-catenin knockout animal model, the interaction of β‐catenin and NF-κB-p65 was abolished. The acute liver injury model reported by Nejak‐Bowen et al. (18) used a high dose of D-Gal (700 mg/kg)/LPS (50 μg/kg) coinjected with TNFα at 2 μg/kg. This treatment caused 100% mortality of WT animals within 6 h (18). In our experiments, we used D-Gal at 400 mg/kg and LPS at 20 μg/kg. Most of the animals survived after 6 h of injury. Early phases of inflammation are characterized by production of proinflammatory mediators, which promotes leukocyte accumulation and causes tissue damage. Following this early proinflammatory phase are phases of inflammatory resolution (23). In these two phases, NF-κB-p65 may function first as a proinflammatory and then an anti-inflammatory factor.
Understanding the modulation of Rspo1 in liver could provide novel therapeutic strategies for hepatic injury. mTOR signaling integrates both intracellular and extracellular signals and serves as a central regulator of cell metabolism, growth, proliferation, and survival (10). mTOR activity and Rspo1 expression are strongly correlated in liver. Deletion of mTOR decreased Rspo1 expression. Consistent with the protective effects of Rspo1 on HIRI, mTOR inhibition potentiated liver injury induced by ischemia/reperfusion (13). Further experiments should explore how mTOR signaling regulates hepatic Rspo1-LGR4 in response to organismal energy levels.
In summary, our studies demonstrate that the Rspo1/LGR4 system in hepatocytes plays a role in hepatocellular protection. Modulation of hepatic Rspo1/LGR4 by mTOR signaling may provide a novel therapeutic strategy for liver damage occurring in association with ischemia/reperfusion, such as liver resection, liver transplantation, or shock.
GRANTS
This research was supported by National Natural Science Foundation of China Grants 81730020 and 81330010; Major National Research Grant of China Grant 2017YFC0908902; and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01-DK-110273-01A1 and R01-DK-112755-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.L. conception and design of research; Z.L., S.L., and J.L. performed experiments; Z.L. and S.L. analyzed data; Z.L., S.L., M.W.M., and W.Z. interpreted results of experiments; Z.L. prepared figures; Z.L. drafted manuscript; Z.L., M.W.M., and W.Z. edited and revised manuscript; Z.L., M.W.M., and W.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jiyao Li for generating the founders of LGR4flox/flox mice.
REFERENCES
- 1.Abe Y, Hines IN, Zibari G, Pavlick K, Gray L, Kitagawa Y, Grisham MB. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med 46: 1–7, 2009. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmon KS, Gong X, Yi J, Thomas A, Liu Q. RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc Natl Acad Sci USA 111: E1221–E1229, 2014. doi: 10.1073/pnas.1323106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlösser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of Wnt/beta-catenin/lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia 12: 326–335, 2010. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494: 247–250, 2013. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ke B, Shen XD, Kamo N, Ji H, Yue S, Gao F, Busuttil RW, Kupiec-Weglinski JW. β-catenin regulates innate and adaptive immunity in mouse liver ischemia-reperfusion injury. Hepatology 57: 1203–1214, 2013. doi: 10.1002/hep.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5: 23–26, 2006. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 7.Kobe B, Deisenhofer J. Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature 366: 751–756, 1993. doi: 10.1038/366751a0. [DOI] [PubMed] [Google Scholar]
- 8.Krönke G, Uderhardt S, Kim KA, Stock M, Scholtysek C, Zaiss MM, Surmann-Schmitt C, Luther J, Katzenbeisser J, David JP, Abdollahi-Roodsaz S, Tran K, Bright JM, Binnerts ME, Akhmetshina A, Böhm C, Distler JH, Joosten LA, Schett G, Abo A. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum 62: 2303–2312, 2010. doi: 10.1002/art.27496. [DOI] [PubMed] [Google Scholar]
- 9.Kuncewitch M, Yang WL, Molmenti E, Nicastro J, Coppa GF, Wang P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock 39: 3–10, 2013. doi: 10.1097/SHK.0b013e3182764fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehwald N, Tao GZ, Jang KY, Sorkin M, Knoefel WT, Sylvester KG. Wnt-beta-catenin signaling protects against hepatic ischemia and reperfusion injury in mice. Gastroenterology 141: 707–718, e1–5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Zhang W, Mulholland MW. LGR4 and its role in intestinal protection and energy metabolism. Front Endocrinol (Lausanne) 6: 131, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Zhang J, Mulholland M, Zhang W. mTOR activation protects liver from ischemia/reperfusion-induced injury through NF-κB pathway. FASEB J 31: 3018–3026, 2017. doi: 10.1096/fj.201601278R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Qian Y, Li L, Wei G, Guan Y, Pan H, Guan X, Zhang L, Lu X, Zhao Y, Liu M, Li D. Lgr4 gene deficiency increases susceptibility and severity of dextran sodium sulfate-induced inflammatory bowel disease in mice. J Biol Chem 288: 8794–8803, 2013. doi: 10.1074/jbc.M112.436204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810, 2004. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 16.Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 8: 108–118, 2011. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustata RC, Van Loy T, Lefort A, Libert F, Strollo S, Vassart G, Garcia MI. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep 12: 558–564, 2011. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatology 57: 763–774, 2013. doi: 10.1002/hep.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, Nigsch F, Xie Y, Roma G, Donovan A, Marti P, Beckmann N, Dill MT, Carbone W, Bergling S, Isken A, Mueller M, Kinzel B, Yang Y, Mao X, Nicholson TB, Zamponi R, Capodieci P, Valdez R, Rivera D, Loew A, Ukomadu C, Terracciano LM, Bouwmeester T, Cong F, Heim MH, Forbes SJ, Ruffner H, Tchorz JS. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol 18: 467–479, 2016. [Erratum in Nat Cell Biol 18: 1260, 2016] doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 20.Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, Schedl A. The angiocrine factor Rspondin3 is a key determinant of liver zonation. Cell Reports 13: 1757–1764, 2015. doi: 10.1016/j.celrep.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 21.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgård R, Clevers H. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389, 2010. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 22.Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138: 4341–4350, 2011. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of Inflammation: What controls its onset? Front Immunol 7: 160, 2016. doi: 10.3389/fimmu.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Loy T, Vandersmissen HP, Van Hiel MB, Poels J, Verlinden H, Badisco L, Vassart G, Vanden Broeck J. Comparative genomics of leucine-rich repeats containing G protein-coupled receptors and their ligands. Gen Comp Endocrinol 155: 14–21, 2008. doi: 10.1016/j.ygcen.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Yi J, Xiong W, Gong X, Bellister S, Ellis LM, Liu Q. Analysis of LGR4 receptor distribution in human and mouse tissues. PLoS One 8: e78144, 2013. doi: 10.1371/journal.pone.0078144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, Kim KA, Liu Y, Levy MD, Berg DJ, Abo A, Funk WD. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology 132: 1331–1343, 2007. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhou WJ, Geng ZH, Spence JR, Geng JG. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 501: 107–111, 2013. doi: 10.1038/nature12416. [DOI] [PMC free article] [PubMed] [Google Scholar]