Abstract
Currently, dual- or triple-drug combinations comprising different vasodilators are the mainstay for the treatment of pulmonary arterial hypertension (PAH). However, the patient outcome continues to be disappointing because the existing combination therapy cannot restrain progression of the disease. Previously, we have shown that when given as a monotherapy, long-acting inhaled formulations of sildenafil (a phosphodiesterase-5 inhibitor) and rosiglitazone (a peroxisome proliferator receptor-γ agonist) ameliorate PAH in rats. Thus, with a goal to develop a new combination therapy, we prepared and characterized poly(lactic-co-glycolic acid) (PLGA)-based long-acting inhalable particles of sildenafil and rosiglitazone. We then assessed the efficacy of the combinations of sildenafil and rosiglitazone, given in plain forms or as PLGA particles, in reducing mean pulmonary arterial pressure (mPAP) and improving pulmonary arterial remodeling and right ventricular hypertrophy (RVH) in Sugen 5416 plus hypoxia-induced PAH rats. After intratracheal administration of the formulations, we catheterized the rats and measured mPAP, cardiac output, total pulmonary resistance, and RVH. We also conducted morphometric studies using lung tissue samples and assessed the degree of muscularization, the arterial medial wall thickening, and the extent of collagen deposition. Compared with the plain drugs, given via the pulmonary or oral route as a single or dual combination, PLGA particles of the drugs, although given at a longer dosing interval compared with the plain drugs, caused more pronounced reduction in mPAP without affecting mean systemic pressure, improved cardiac function, slowed down right heart remodeling, and reduced arterial muscularization. Overall, PLGA particles of sildenafil and rosiglitazone, given as an inhaled combination, could be a viable alternative to currently available vasodilator-based combination therapy for PAH.
Keywords: arterial remodeling, inhalation delivery, pulmonary arterial hypertension, right ventricular hypertrophy, rosiglitazone, sildenafil
INTRODUCTION
The treatment outcome of pulmonary arterial hypertension (PAH), a severe form of pulmonary vascular disease, has been disappointing, and thus patient survival rate continues to be ~80 and ~50% after 1 and 5 yr of diagnosis, respectively (9). A better understanding of PAH pathogenesis over the past two decades has spurred the development of more effective therapies for PAH, and thus various types of drugs are commercially available and clinically used for the treatment of this incurable disease (27). Though relatively newer molecules function by interrupting an array of signaling pathways, all existing drugs are predominantly vasodilators. They have no direct effect on pulmonary arterial remodeling, which leads to right ventricular hypertrophy (RVH), the major cause of death from PAH (49). Dual- or triple-vasodilator combinations, given either as a sequential or up-front regimen, although improving patient morbidity and survival rate, have been of limited help in reversing progressive deterioration of pulmonary arterial remodeling (11, 30, 32).
Besides orally administered vasodilators, two approved PAH medications, treprostinil and iloprost, are currently available as inhalable solutions, which are currently used to treat only adult patients with PAH (63, 64). Inhaled iloprost or treprostinil is used to prevent acute exacerbation of PAH, but inhaled forms of these drugs are not used as replacements for their intravenous or oral counterparts. Furthermore, multiple dosing a day (the dosing frequencies of treprostinil and iloprost are 4 and 6–9 times a day, respectively), cumbersome inhalation devices, and side effects such as bronchoconstriction, cough, and increased airway reactivity seriously restrict their clinical applications (63, 64). In addition, because of the safety concerns, inhaled anti-PAH medications are not recommended for pediatric patients who are 8 yr old or younger, an important cohort of patients with PAH (24, 56).
To address the limitations of multiple dosing for inhaled anti-PAH drugs, we previously demonstrated that dosing interval can be extended and thus the dosing frequency for anti-PAH medications can be reduced by administering the drugs as inhalable sustained-release particulate formulations (16, 18, 39, 40). We have shown that inhalable drug-laden particles, given either in the form of biodegradable polymeric particles or liposomes, can elicit pulmonary-specific effects of anti-PAH drugs at a reduced dosing frequency (15–17, 40). The inhaled formulations of various anti-PAH drugs including prostaglandin E1, fasudil, long-acting nitric oxide donors, and superoxide dismutase, when formulated as polymeric or lipidic particles, can minimize drops in systemic arterial pressure by restricting the drugs’ vasodilatory effects within the pulmonary vasculature. In a recent study, we showed that by formulating it as inhalable particles, rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist that is reported to inhibit pulmonary arterial remodeling and arrest PAH progression (14, 28, 33, 41, 54), can be repurposed for the treatment of PAH (46). In a separate study, we demonstrated that inhaled poly(lactic-co-glycolic acid) (PLGA) particles of sildenafil, an orally active phosphodiesterase type 5 inhibitor that is frequently used as monotherapy or in combination therapy for PAH (13), improve pulmonary hemodynamics in PAH rats when given at a reduced dose and longer dosing intervals (48).
As a continuation of the above two studies (46, 48), wherein we assessed the feasibility of inhaled particulate formulations of rosiglitazone and sildenafil for the treatment of PAH, here we tested the hypothesis that a combination of sildenafil and rosiglitazone, when given as prolonged-release formulations via the pulmonary route, improves pulmonary hemodynamics, RVH, and pulmonary arterial remodeling. As such, we first prepared PLGA particles of sildenafil and rosiglitazone and assessed them for their suitability for administration via the pulmonary route. After intratracheal (IT) administration of 1) plain drugs, 2) PLGA particles of either sildenafil or rosiglitazone, and 3) combination of PLGA particles of sildenafil plus rosiglitazone to PAH rats for 3 wk, we measured pulmonary hemodynamics and assessed the effect of the formulations on RVH and pulmonary arterial remodeling upon morphometric analysis.
MATERIALS AND METHODS
Materials.
PLGA polymers (lactic-to-glycolate ratios of 50:50 and 75:25; inherent viscosity 0.55–0.75 dl/g) were purchased from Lactel Absorbable Polymers (Birmingham, AL). Sildenafil citrate and rosiglitazone maleate were from Biotang (Lexington, MA) and Cayman Chemical (Ann Arbor, MI), respectively. Male Sprague-Dawley rats (250–350 g) were obtained from Charles River Laboratories (Wilmington, MA). All other chemicals were of HPLC grade and were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation and characterization of PLGA particles of sildenafil and rosiglitazone.
Sildenafil- and rosiglitazone-loaded microparticles were prepared according to our previously established water-in-oil-in-water (W1/O/W2) double emulsion-solvent evaporation method (46, 48). In short, 0.5 ml of an internal aqueous phase (W1) containing rosiglitazone maleate (20 mg/ml) or sildenafil citrate (20 mg/ml) in a mixture of methanol and double-deionized water (20:80) plus 0.5% polyethyleneimine (PEI) in water was first emulsified in a PLGA polymer solution in dichloromethane (organic phase) using a Branson Sonifier 450 (Branson Ultrasonics, Danbury, CT). The organic phase for preparing sildenafil and rosiglitazone particles had 250 mg of PLGA 75:25 polymer in 5 ml of dichloromethane and 100 mg of PLGA 50:50 polymer in 5 ml of dichloromethane, respectively. A double emulsion was prepared by homogenizing the primary (W1/O) emulsion with 0.5% wt/vol polyvinyl alcohol solution (electroactive polymer, W2). The double emulsion was then stirred for 8 h at room temperature for evaporation of the organic phase and hardening of the particles. The polymeric particles were then washed three times with water and lyophilized for 48 h to obtain free-flowing powdered formulations.
Both sildenafil- and rosiglitazone-loaded particles were characterized for their size, zeta potential, and encapsulation efficiency. The volume-based mean diameter and particle size distribution of the formulations were determined in a Malvern Mastersizer 2000 (Malvern Instruments, Malvern, UK) particle size analyzer. Freeze-dried particles (~15 mg) were dispersed in deionized water using a Hydro 2000MU sample dispersion unit and pumped into the particle size analyzer. The zeta potential was measured using a Nano ZS90 Zetasizer (Malvern Instruments) after dispersing equal amounts of particles in 1× PBS buffer. The entrapment efficiencies of the formulations were determined directly by dissolving ~5 mg of freeze-dried particles in a 1:1:1 mixture of methanol-dimethyl sulfoxide-dichloromethane. The UV absorbance was measured at 311 and 293 nm for sildenafil and rosiglitazone, respectively, using a spectrophotometer (Hewlett Packard, Palo Alto, CA). The concentrations in the dissolved particles were obtained from a calibration curve of absorbance versus concentration of sildenafil citrate or rosiglitazone maleate. The entrapment efficiency was calculated using the following equation: entrapment efficiency (%) = (amount of drug in particles/amount of drug originally added) × 100.
Effect of the formulations on pulmonary hemodynamics, cardiac function, and arterial remodeling.
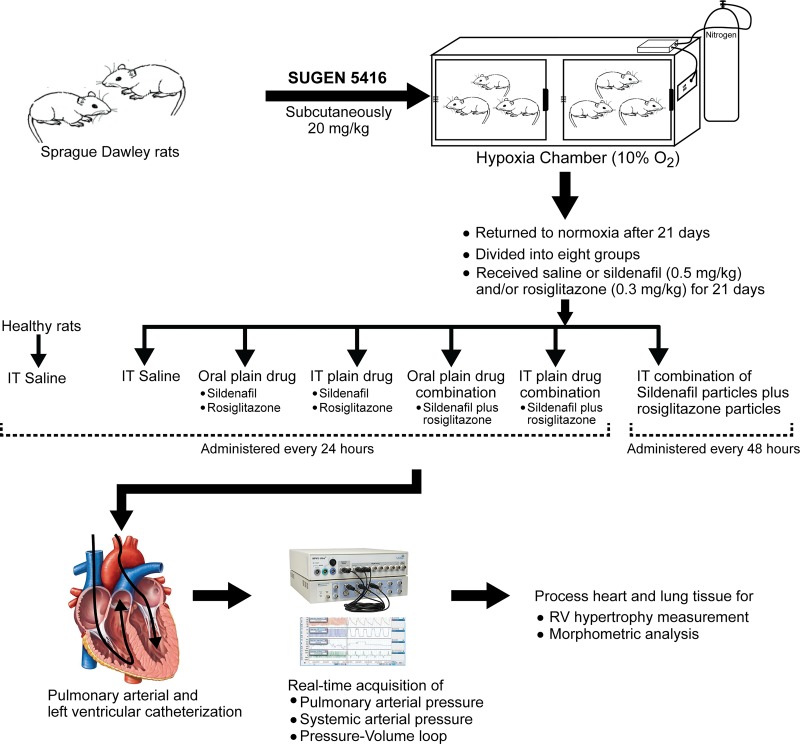
The long-term efficacy studies were conducted in Sugen-hypoxia-induced PAH rats as described previously (42, 55, 66; Fig. 1). For development of PAH, adult male Sprague-Dawley rats (200–225 g; Charles River) were given a single subcutaneous injection of Sugen 5416 (20 mg/kg; Bio-Techne, Minneapolis, MN) (composition: 20 mg/kg Sugen 5416 and 0.5% carboxymethyl cellulose, 0.9% NaCl, 0.4% Tween 20, and 0.9% benzyl alcohol wt/vol in water), and then Sugen 5416-treated rats were kept in a hypoxia chamber (BioSpherix, Lacona, NY) at 10% oxygen for 3 wk. Animals had free access to food and water. On the 22nd day, rats were removed from the hypoxic chamber and housed in normoxia for an additional 3 wk. During these 21 days, when rats were housed in normoxia, PAH rats were divided into eight groups to receive 1) IT 100 µl saline, once a day; 2) oral plain sildenafil, once a day; 3) oral plain rosiglitazone, once a day; 4) IT plain sildenafil, once a day; 5) IT plain rosiglitazone, once a day; 6) oral plain sildenafil and plain rosiglitazone combination, once a day; 7) IT plain sildenafil and plain rosiglitazone combination, once a day; or 8) IT sildenafil particle and rosiglitazone particle combination, every 48 h (Fig. 1). The doses of sildenafil and rosiglitazone were 0.5 and 0.3 mg/kg, respectively. One group of healthy rats was used as the sham group. IT administration was performed using a Penn Century MicroSprayer (Penn Century, Wyndmoor, PA) upon illuminating the trachea using a rat laryngoscope, as reported previously (48).
Fig. 1.
Protocol for the development of pulmonary arterial hypertension in rats: study design and various treatment groups. IT, intratracheal; RV, right ventricle.
Measurement for pulmonary hemodynamics and cardiac functional parameters.
To measure mean pulmonary arterial pressure (mPAP) and mean systemic arterial pressure (mSAP), PAH rats were anesthetized by intramuscular administration of ketamine and xylazine, and the ventral neck area of the rats was shaved and then cleaned using ethyl alcohol. To measure mPAP, a polyvinyl catheter (PV-1, 0.28-mm internal diameter, Tygon) was inserted into the right jugular vein to reach the pulmonary artery via the right ventricle. For mSAP, a Millar pressure-volume catheter (SPR-868; Millar, Houston, TX) was inserted into the left ventricle through the right carotid artery. The data were recorded in a PowerLab 16/30 system (ADInstruments, Colorado Springs, CO) equipped with Millar Pressure-Volume System (MPVS) Ultra (Millar) using LabChart Pro 7.0 software (ADInstruments). mPAP was measured using MEMSCAP SP844 physiological pressure transducers (MEMSCAP, Skoppum, Norway) and bridge amplifier (19), whereas mSAP was measured using MPVS Ultra. The signals were continuously recorded for ~30 min (Fig. 1). A cardiac pressure-volume relationship (P-V loop) in the left ventricle was assessed to obtain hemodynamic parameters using a pressure-volume analysis program of the LabChart Pro 7.0 software. mSAP and cardiac output were measured by analyzing the P-V loops. Cardiac index (CI), the weight-normalized cardiac output, was also obtained by dividing the cardiac output by the body weight of the animal (1, 67). The total pulmonary vascular resistance (TPVR) was calculated by dividing the mPAP by the respective cardiac output (34, 50, 67, 69). After each hemodynamic study, the rats were euthanized, and the lungs and hearts were collected for morphometric analyses and calculation of RV weight-to-LV plus septum weight ratio (RV/LV+S), respectively. Animal studies were performed by following the NIH’s Guideline for the Care and Use of Laboratory Animals under an approved protocol (AM-10012) from Texas Tech University Health Sciences Center Animal Care and Use Committee.
Preparation of lung samples for morphometric studies.
After measuring the pulmonary hemodynamics, lung tissues were collected and fixed with 10% formalin solution; the chest was opened, and heart and lungs were exposed. The left lung was inflated and fixed with 10% formalin under 30-cmH2O pressure (7). The heart and lungs were then removed en bloc from the body and stored in paraformaldehyde for a day. To measure RVH, the heart was removed from the block. Afterward, the left lung was transferred to 70% ethanol in water before embedding in paraffin blocks to be used for morphometric analyses.
Assessment of RVH and immunostaining for morphometric analysis.
RVH was measured from RV/LV+S as previously described (47). Lung sections (5 µm) from formalin-fixed and paraffin-embedded left lungs were deparaffinized with CitriSolv (Thermo Fisher Scientific, Pittsburgh, PA) and subsequently rehydrated with a series of gradient ethanol in water. Sections were boiled in antigen-unmasking solution, blocked using 10% horse serum (Vector, Burlingame, CA), incubated overnight first with a mouse monoclonal α-smooth muscle actin (α-SMA) antibody, clone 1A4 (Sigma-Aldrich), and then with peroxidase-conjugated anti-mouse/anti-rabbit secondary antibody (R.T.U. Vectastain Universal; Vector), and finally stained with peroxidase-sensitive ImmPACT diaminobenzidine substrate (Vector). Similarly, von Willebrand factor (vWF) was stained with rabbit polyclonal anti-vWF antibody (Sigma-Aldrich) and peroxidase-sensitive ImmPACT VIP substrate (Vector; 65). Nuclei were counterstained with methyl green (Vector), and slides were viewed at ×200 using an Aperio ScanScope system (Leica Biosystems, Buffalo Grove, IL). A positive pixel count algorithm was created using Aperio ImageScope software (Leica Biosystems) to determine the degree of muscularization and measure the medial wall thickness (MWT).
Quantitation of the degree of muscularization and arterial MWT.
For the quantitation of the muscularized vessels, we counted the number of blood vessels that were <50 µm in diameter and had a positive α-SMA stain in 10 fields of view (×10 magnification). Muscularized pulmonary arteries and arterioles that occupied >50% of the diameter of the lumen and intima of the vessels were considered as muscularized arteries. For MWT, four measurements of the perpendicular lumen radius and medial wall were taken for small muscularized arteries (<50 µm). MWT was expressed as the average MWT divided by the average vessel radius. The α-SMA staining was evaluated by an investigator blinded to the treatment groups.
Collagen analysis.
The slides containing slices of the lungs were deparaffinized and stained using a Masson’s trichrome staining kit (HT15-1KT; Sigma-Aldrich) to visualize and quantify collagen deposition around the pulmonary arteries. Briefly, lung sections (100 µm) were deparaffinized with CitriSolv (Thermo Fisher Scientific) and rehydrated in graded ethanol followed by deionized water. Sections were then treated with preheated Bouin solution for 15 min followed by sequential treatment with Weigert’s hematoxylin, Biebrich scarlet-acid fuschin, phosphotungstic/phosphomolybdic acid, aniline blue, and 1% acetic acid solutions. Finally, stained sections were rinsed in tap water, dehydrated using ethanol, and mounted in xylene. Stained sections were imaged by the Aperio ScanScope system (Leica Biosystems), and collagen deposition was quantitated using a positive pixel count algorithm using Aperio ImageScope software (Leica Biosystems) by an investigator blinded to treatment groups.
Statistical analyses.
All results are presented as means ± SE and were analyzed by one-way ANOVA followed by Fisher’s least significant difference multiple-comparison test using OriginPro 2016 (OriginLab, Northampton, MA); P < 0.05 is considered to be statistically significant.
RESULTS
PLGA particles of sildenafil and rosiglitazone are optimal for deep-lung deposition.
Volume-based diameters of sildenafil and rosiglitazone particles, prepared separately for each individual drug, were 4.07 ± 0.85 and 4.32 ± 0.86 µm, respectively (Table 1). The size of an inhalable particulate drug formulation dictates its deposition patterns within the respiratory tract (29). Deposition of drugs in the peripheral lung enhances the penetration and retention of the inhaled formulations and thus produces maximum therapeutic benefits. Both sildenafil and rosiglitazone particles show an optimum physical diameter (~5 µm) that is optimal for deposition in the alveolar region upon inhalation (4, 22, 29). The drug loading efficiencies of these formulations were also high (76.86 ± 4.50% for sildenafil and 84.19 ± 5.70% for rosiglitazone). Increased drug loading helps reduce the total amount of formulations to be delivered for a given dose and thus minimizes excipient-induced complications in the lungs. These formulations continued to release the drugs for over 24 h.
Table 1.
Physicochemical and release characteristics of sildenafil and rosiglitazone particles
| Formulation | Sildenafil Particles | Rosiglitazone Particles |
|---|---|---|
| Polymer type (PLGA) | 75:25 | 50:50 |
| Volume-based diameter, µm | 4.07 ± 0.85 | 4.32 ± 0.86 |
| Zeta potential, mV | 5.32 ± 1.97 | −4.90 ± 5.51 |
| Drug entrapment efficiency, % | 84.19 ± 5.70 | 84.19 ± 5.70 |
| In vitro release of drug in a simulated lung fluid | ||
| Over 8 h | 41.00 ± 3.89 | 67.81 ± 16.86 |
| Over 24 h | 51.95 ± 1.37 | 87.85 ± 6.69 |
Values are means ± SE. PLGA, poly(lactic-co-glycolic acid).
Combination of sildenafil and rosiglitazone particles elicited maximal reduction in mPAP but minimal reduction in mSAP.
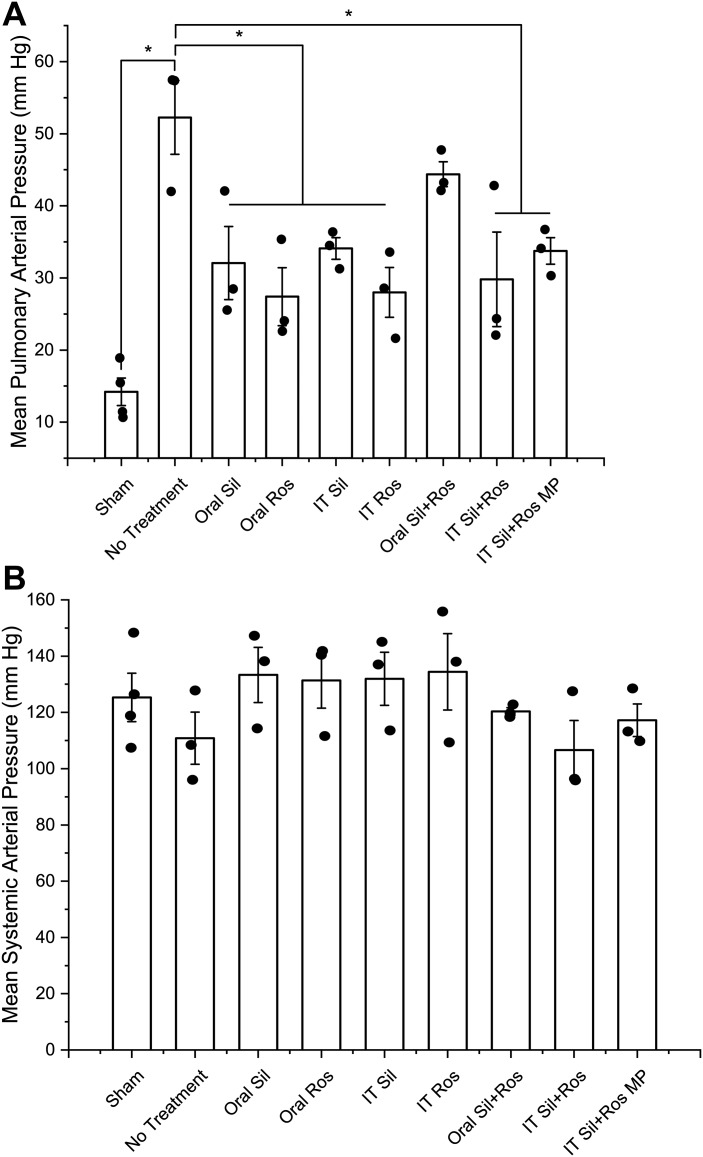
The mPAP of control animals (sham animals) was the same as the physiological value (14.2 ± 1.9 mmHg, Fig. 2A). In vehicle-treated PAH animals, the mPAP was 3.7-fold of the physiological mPAP (52.3 ± 5.1 mmHg), suggesting that the animals had a severe form of PAH. However, PAH rats that received either sildenafil or rosiglitazone, given once a day for 21 days via the oral or IT route, showed a 34.8–47.6% reduction in mPAP compared with PAH rats treated with saline (Fig. 2A). Once-a-day treatment with the combination of plain sildenafil plus rosiglitazone, given IT, showed a 43% reduction in mPAP, and once-every-48-h treatment with the combination of particles of sildenafil and rosiglitazone produced a 35.4% reduction in mPAP compared with saline-treated rats. The IT combination of plain drugs, although given twice as frequently as the particle combination via the same route, showed slightly more reduction in mPAP than the IT particle combination did, but the difference was not statistically significant. The extent of reduction in mPAP induced by oral plain drug combination was not statistically significant compared with that in saline-treated PAH rats. The mSAPs in various treatment groups were between 106.6 ± 10.1 and 134.5 ± 13.6 mmHg, indicating that none of the treatments caused drops in systemic blood pressure, a well-known side effect of PAH medications (Fig. 2B). Although the mSAP in PAH rats (123.26 ± 17.1 mmHg) was slightly greater than that in healthy rats (119.48 ± 19.9 mmHg), there was no statistically significant difference between control and test animals, and thus PAH caused no increase in mSAP.
Fig. 2.
Effect of plain drugs or formulations on mean pulmonary arterial pressure (A) and mean systemic arterial pressure (B). Data represent means ± SE. IT, intratracheal; MP, microparticles; Ros, rosiglitazone; Sil, sildenafil; Data were analyzed using one-way ANOVA followed by Fisher’s least significant difference test; n = 3–4, *P < 0.05.
Combination of sildenafil and rosiglitazone particles improved cardiac function.
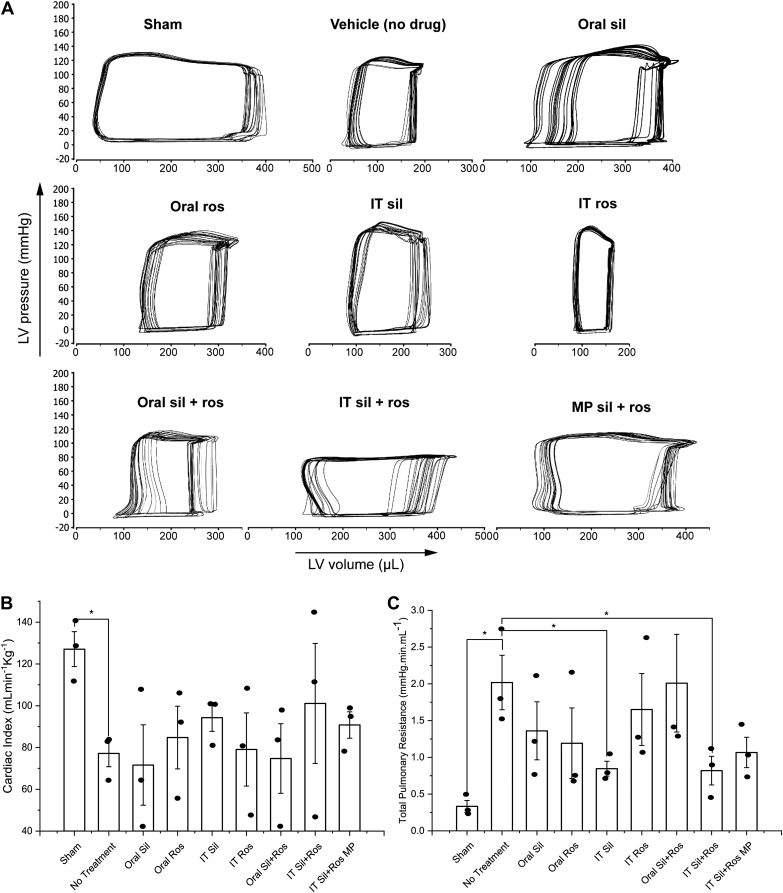
Representative P-V loop diagrams from each of the 9 treatment groups, obtained from 20 left ventricular P-V loops of the animals (Fig. 3A), suggest that the stroke volume (the horizontal length of the loop) of the healthy animals was substantially larger than that of the saline-treated PAH rats. Saline-treated PAH rats had the smallest stroke volume, as evidenced by the narrowest horizontal width of the loop. Treatment of PAH rats for 21 days with a single or combination therapy with sildenafil and/or rosiglitazone, either in plain form or in particulate formulation, appears to improve the stroke volume of PAH rats.
Fig. 3.
A: representative pressure-volume loops from each treatment group. B and C: efficacy of plain drugs or formulations in improving cardiac functional parameters: cardiac index (B) and total pulmonary vascular resistance (C). Data represent means ± SE. IT, intratracheal; LV, left ventricle; MP, microparticles; Ros, rosiglitazone; Sil, sildenafil. Data were analyzed using one-way ANOVA followed by Fisher’s least significant difference test; n = 3–4, *P < 0.05.
To assess the effect of the treatments in ameliorating functional deterioration of the heart, we measured CI, the body weight-normalized function of cardiac output in PAH rats (36, 57). A 60.7% reduction in mean CI in saline-treated PAH rats compared with sham animals suggests a major deterioration of cardiac output due to the development of PAH (Fig. 3B). The effect of oral sildenafil, oral rosiglitazone, and IT rosiglitazone on mean CI was rather modest, but IT sildenafil improved the CI by 22.3% compared with saline-treated PAH rats. IT plain drug combination caused a 31% improvement in mean CI; the variability was rather high with a relative standard error (RSE) of ~28.4%. Oral combination therapy reduced the mean CI by 3.1%. However, IT sildenafil plus rosiglitazone particles, administered every 48 h, produced a 17.7% improvement in mean CI compared with saline-treated groups, and the variation in this treatment group was rather small (RSE, ~7%).
To evaluate the effects of various treatments in reducing pulmonary vascular resistance, a major indicator of PAH-associated vascular remodeling, we assessed TPVR for different groups (3, 31). Saline-treated PAH rats showed a 6.1-fold increase in TPVR compared with sham animals, an indication of abnormally high mPAPs observed in saline-treated PAH rats (Fig. 3C). Despite a major reduction in mean TPVR in PAH rats treated with oral sildenafil and oral and IT rosiglitazone, within-group variability was very high (RSE > 29%). The TPVRs in IT sildenafil- and IT plain drug combination-treated PAH rats were 58% lower than that in saline-treated PAH rats. However, unlike other treatment groups, within-group variation was small. Rats treated with the combination of the particles of the two drugs showed a 47.1% reduction in TPVR with low intersubject variability (RSE, 19.4%) compared with the saline-treated PAH rats.
Combination of sildenafil and rosiglitazone particles reduced RVH in PAH rats.
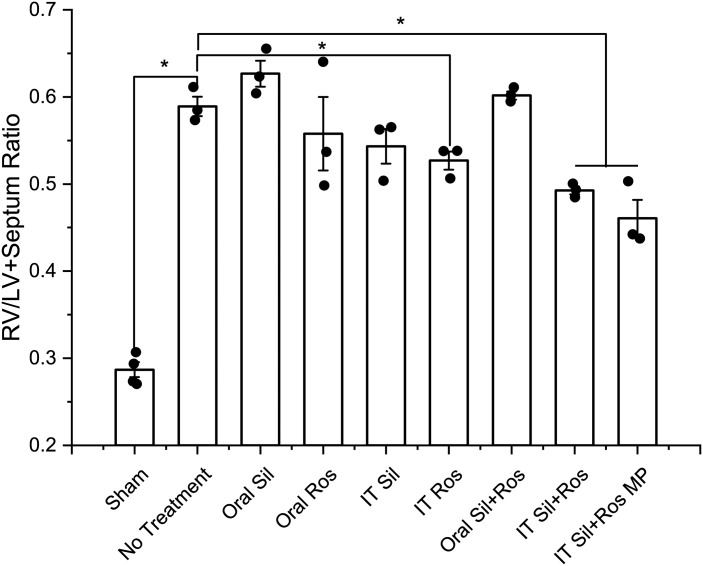
We evaluated the effects of different treatments in reducing the extent of RVH, the major contributor to the death of 80% of patients with PAH (38). An increase in RV/LV+S indicates right heart enlargement (1). Sugen 5416-hypoxia caused significant RVH in vehicle-treated PAH rats as evidenced by a twofold increase in RV/LV+S compared with that of the healthy rats (Fig. 4). Once-a-day treatment with IT rosiglitazone showed a 10.5% reduction in RV/LV+S. Oral plain sildenafil, given either as a single drug or in combination with oral plain rosiglitazone, caused no statistically significant changes in RV/LV+S compared with that of the vehicle-treated controls. Compared with the vehicle-treated PAH rats, RV/LV+S in the IT sildenafil-treated group declined by 5.3%. IT combination of plain sildenafil and rosiglitazone showed a statistically significant reduction (16.4%) in RV/LV+S. However, combination therapy with sildenafil plus rosiglitazone particles showed the greatest reduction (21.8%) in RV/LV+S, which was statistically significant compared with that of the vehicle-treated PAH rats.
Fig. 4.
Effect of various treatments on right ventricle (RV)-to-left ventricle (LV) plus septum ratio, a direct measure of right heart remodeling in pulmonary arterial hypertension. Data represent means ± SE. IT, intratracheal; MP, microparticles; Ros, rosiglitazone; Sil, sildenafil. Data were analyzed by one-way ANOVA followed by Fisher’s least significant difference test; n = 3–4, *P < 0.05.
IT combination of sildenafil and rosiglitazone improved pulmonary arterial remodeling.
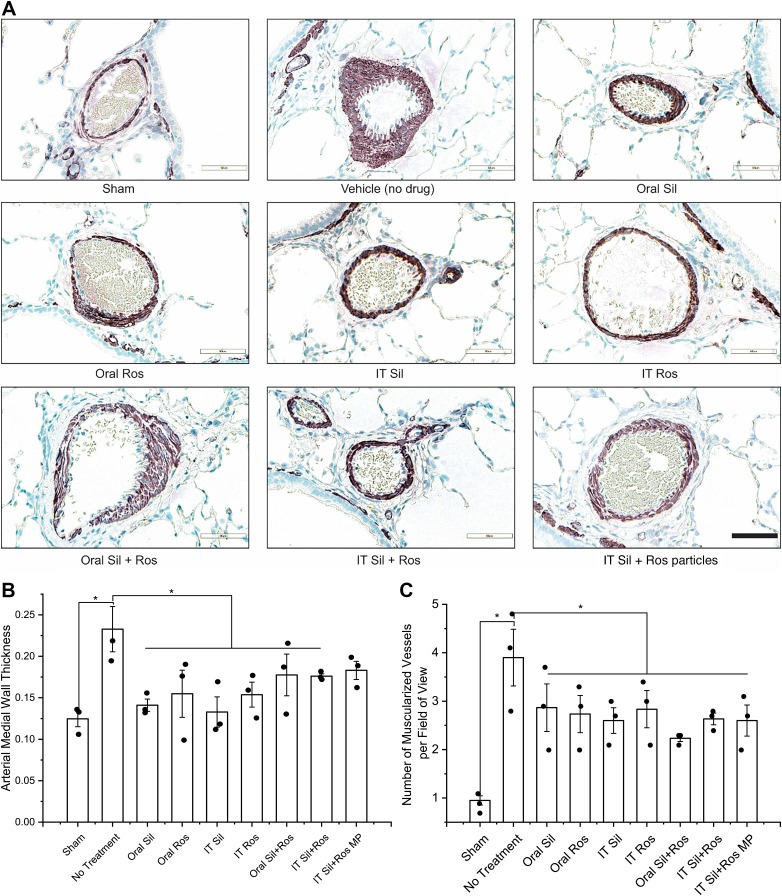
In PAH, muscularization and remodeling of both extracellular matrices and pulmonary arteries cause narrowing of the pulmonary arteries and arterioles (25). Thus, we immunostained vWF and α-SMA in the lungs and used them as markers for endothelial and smooth muscle cell activation, respectively (Fig. 5A). The vascular walls of the pulmonary arteries of the saline-treated PAH rats were thicker than those of sham animals (Fig. 5A), pointing to the arterial remodeling due to PAH. When treated with IT plain sildenafil, IT plain rosiglitazone, IT combination of plain drugs, and IT combination of particles, arterial thickening declined, pointing to the preventive effects of the formulations on endothelial and smooth muscle proliferation (Fig. 5A).
Fig. 5.
A: representative microphotographs of the muscularized arteries of rats treated with plain drugs and formulations. B and C: effect of the drugs or formulations on arterial medial wall thickening (B) and extent of muscularized pulmonary arteries (C). IT, intratracheal; MP, microparticles; Ros, rosiglitazone; Sil, sildenafil. Data were analyzed using one-way ANOVA followed by Fisher’s least significant difference test; n = 3–4, *P < 0.05.
We calculated mean arterial thickening (MWT) to quantify the degree of arterial thickening in different treatment groups (Fig. 5B). The MWT in vehicle-treated PAH lungs was ~2-fold greater than that in sham animals. Except in the IT particle combination group, MWTs in all treatment groups were smaller than those in the vehicle-treated group, and the differences between the groups were statistically significant. Compared with the vehicle-treated group, the reduction in MWTs in the once-every-48-h treatment (21.3%) group was no different from IT once-a-day treatment with plain drug combination (24.45%). However, statistically, there was no difference in the MWTs of various treatment groups.
We also quantified the number of occluded vessels present in each large microscopic field of the stained lung sections of different treatment groups (Fig. 5C). The number of occluded vessels in vehicle-treated PAH rats was 4.1 times greater than that in sham rats. Compared with the vehicle-treated group, drug treatment caused a significant decrease in the number of occluded vessels across all the treatment groups. Similar to the MWT data, there were no statistically significant differences among the seven groups that received various treatments with drugs or formulations.
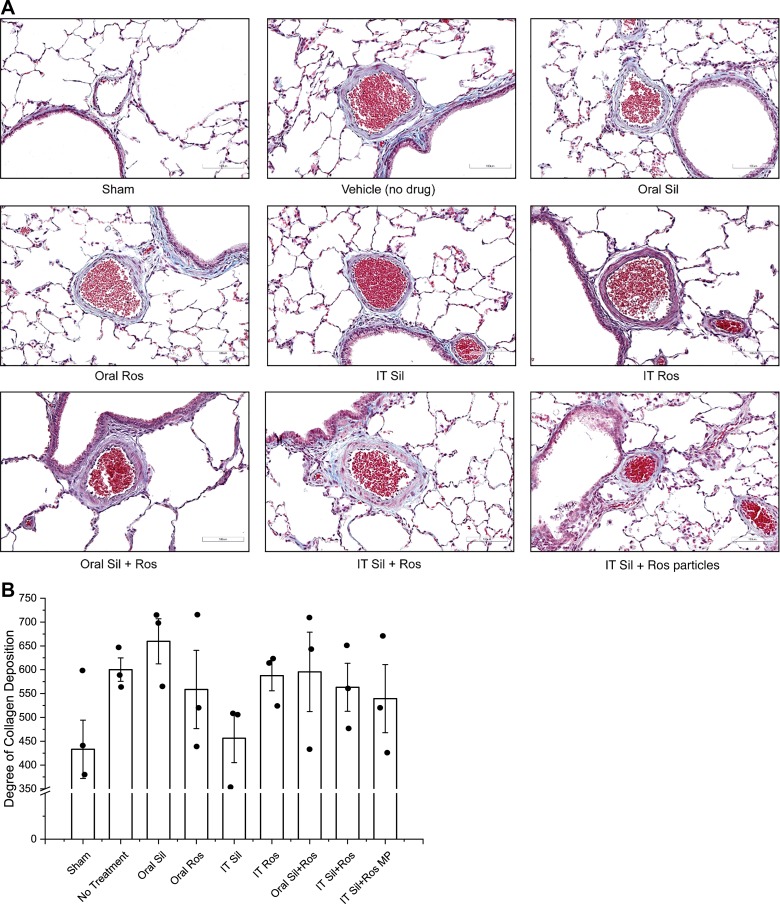
Sildenafil and/or rosiglitazone reduced arterial collagen deposition.
A major pathophysiological feature of PAH is the deposition of extracellular matrix proteins, such as collagen fibers, in the pulmonary arteries (52, 68). Thus, we assessed the effect of the formulations on collagen deposition. In PAH rats, the deposition of collagen was much higher than that in sham animals (Fig. 6A). However, collagen deposition declined in all drug-treated groups, except oral sildenafil, although the extent of collagen deposition in various treatment groups was not statistically different (Fig. 6B). However, in saline-treated PAH animals, collagen deposition was 38.6% higher than in healthy animals, suggesting the presence of progressive PAH. However, IT plain sildenafil treatment caused an ~24.0% reduction, and the combination of particles caused a 10.1% reduction, in collagen deposition compared with saline-treated PAH rats.
Fig. 6.
A: representative micrographs of the pulmonary arteries stained for the presence or absence of collagen. B: extent of collagen deposition. Data represent means ± SE; n = 3–4. IT, intratracheal; MP, microparticles; Ros, rosiglitazone; Sil, sildenafil.
DISCUSSION
Currently, sildenafil, although used extensively as a vasodilator for PAH management, cannot adequately improve pulmonary hemodynamics and slow PAH progression (51). First, dose-related adverse events limit the use of sildenafil in pediatric patients with PAH (51, 62). Systemic adverse events often preclude escalation of sildenafil dose in patients with PAH who become unresponsive to this drug. As such, these patients are left with the options of dual- or triple-combination therapies (12, 13). Recently, we have shown that an inhalable low-dose sustained release formulation of sildenafil elicits hemodynamic relief in PAH rats for an extended period, ~6 h (48). Rosiglitazone is known to be effective in preventing right heart remodeling, the major cause of PAH-related mortality. While vasodilator-based combination therapy is the mainstay for PAH management (12), here we evaluated the long-term effects of an inhaled combination therapy, given as a sustained-release inhalable formulation that can accomplish major treatment goals: to provide hemodynamic relief, to improve arterial remodeling, and to reduce RVH. More to this point, patients with PAH often fail to comply with or adhere to a given treatment regimen because of the requirement of multiple dosing a day. Indeed, too many doses a day can seriously erode patients’ freedom and lead to noncompliance. As shown above and discussed below, the PLGA formulations of sildenafil and rosiglitazone that we have developed can potentially be administered every other day instead of every day or multiple times a day because the formulations, given every 48 h, are as efficacious in ameliorating various PAH pathologies as the plain drugs given every day.
We used a Sugen-hypoxia rat model of PAH because it most closely resembles human PAH. This model exhibits high mPAP, manifests vascular changes similar to those of human PAH, and protects animals from non-PAH-associated mortality (6, 53). The highest daily dose of sildenafil for adult patients with PAH is 60 mg, equivalent to 5.4 mg·kg−1·day−1 in rats as per US Food and Drug Administration guidance on interspecies dose conversion (60, 61). In this study, the dose for sildenafil particles was 0.25 mg·kg−1·day−1, which is <1/20th of the approved dose in humans. Similarly, the rat-equivalent dose for rosiglitazone (4 mg/day) in patients with diabetes is 0.36 mg·kg−1·day−1, and the dose of rosiglitazone particles was 0.15 mg·kg−1·day−1, one-half of the regular human dose for diabetes (59). Thus, the novel aspect of this study was the evaluation of the long-term efficacy of a low-dose and pulmonary-specific combination therapy comprising a vasodilator and an inhibitor of vascular remodeling in PAH.
The physicochemical properties of both sildenafil and rosiglitazone particles are identical and suggest that these particles are most likely to be distributed in the alveolar region of the lungs upon inhalation (4, 22, 29). High drug loading efficiency, observed in the case of both sildenafil and rosiglitazone particles, is a major advantage because it eases drug administration with the microsprayer and reduces IT administration-associated trauma in rats (8). Systemic and pulmonary hemodynamics of the Sugen 5416-hypoxia rats that did not receive any of the drugs suggest development of moderate to severe forms of PAH as previously reported (42, 55). Once-a-day administration of a single drug, either given orally or IT for 21 days, slowed the progression of PAH, as evidenced by the significantly reduced mPAPs in treatment groups compared with the vehicle-treated control group. Lack of effects in the oral plain drug combination group can be attributed to the poor absorption from the gastrointestinal tract. Combination particles, although given every 48 h, were as effective as the plain drug combination given once a day IT because PLGA particles released the drugs slowly but continuously over a longer period on the highly absorptive alveolar surface (20, 44, 46, 48). The systemic arterial pressure remained unaltered perhaps because the doses of the drugs were too small to produce a measurable systemic effect. The mSAPs in healthy sham rats were very close to those in PAH rats, suggesting that PAH development had no effect on the systemic pressure. Together, hemodynamic data suggest that particles of sildenafil and rosiglitazone can effectively restrain disease progression with less frequent dosing.
We observed a deterioration of cardiac function in the PAH rats that received no drugs. Representative P-V loops and CI from different treatment groups suggest that Sugen-hypoxia produced severe PAH that significantly limited cardiac output as previously documented (1, 2, 37, 42). IT plain sildenafil or IT plain drug combination appeared to restore the CI slightly although there was a higher interanimal variability. Combination particles showed a moderate improvement in CI with low intersubject variability. High pulmonary vascular resistance (>3 mmHg·l−1·min−1) is an integral part of the hemodynamic definition of PAH that distinguishes PAH from other forms of pulmonary hypertension (23). We calculated TPVR, as an indirect measure for pulmonary vascular resistance, to assess disease progression as well as the effect of different treatments (5). Similar to CI, a significant increase in TPVR in the vehicle-treated group suggests worsening of PAH. IT sildenafil, IT plain drug combination, and IT combination particles reduced TPVR to half of that observed in saline-treated PAH rats. Reduction in TPVR produced by combination particles can be attributed to the sustained pulmonary vasodilation and inhibition of vascular remodeling by sildenafil and rosiglitazone at the local pulmonary vasculature (7, 21, 28, 46, 48). Altogether, our study indicates a functional deterioration of the cardiac parameters in Sugen-hypoxia-induced PAH and that sildenafil plus rosiglitazone improves the cardiac functional capacity in diseased rats.
RV/LV+S is used to evaluate PAH-associated right heart remodeling in preclinical models (1, 20, 42). All but the oral plain drug combination treatments caused a statistically significant reduction in PAH-induced RVH, as reflected by the elevated RV/LV+S. Although the effects within the drug-treated groups were not statistically significant, the greatest reduction in RV/LV+S being observed in the IT combination particle group suggests that prolonged and pulmonary-preferential effects of the drugs are more effective in reducing RVH.
In PAH, rapid proliferation of the smooth muscle cells of the medial layer along with endothelial dysfunction causes narrowing of the small pulmonary arterioles (25). Furthermore, progression of PAH involves proliferation of both endothelial and smooth muscle cells in the pulmonary arteries (26, 35, 45). Qualitative micrographs and quantitative morphometry of the lung tissues indicate that single drugs or plain drug combinations were effective in preventing arterial thickening; combination particles, administered at a longer interval, inhibited arterial thickening to the same extent although plain drug versus particle combination-induced effects were not significantly different. The number of muscularized vessels in drug-treated groups versus saline-treated rats confirmed that particles, given at the same dose but at a longer dosing interval (24 vs. 48 h), produced similar improvements in arterial muscularization. Thus, echoing the observed hemodynamic effects, morphometric analyses suggest that the PLGA formulations of these drugs can be given at a longer interval to produce similar effects in preventing arterial muscularization and thickening. Collagen accumulation is a key contributor to the pulmonary arterial stiffening and remodeling in PAH (10, 43, 58). In the present study, a moderate increase in the mean collagen content in the vehicle-treated group compared with that in the healthy rats suggests that hyperactive collagen synthesis was implicated in disease progression. Hemodynamic improvement caused by the treatments should reduce collagen deposition, but we found no statistically significant differences between treatment and control groups. Lower collagen content in IT sildenafil and combination particle groups compared with the untreated controls indicates a trend toward slowing of PAH progression. A long-term study for 12 wk would perhaps help determine the effect of this drug combination on collagen deposition.
However, the efficacy data may not be on par with the safety of the formulation, which should be assessed after chronic administration of the formulations to both control and PAH rats. Rosiglitazone-induced changes, for example, in the liver should be carefully evaluated, although the dose of this drug used here is much smaller than the US Food and Drug Administration-approved dose. We previously evaluated the safety of the inhalable sildenafil particles after a single dose in healthy rats (46, 48), but a long-term safety study of the proposed combinations has not yet been conducted. More importantly, a survival study using PAH animals would provide further credence to the assumption that the combination of sildenafil and rosiglitazone improves both cardiac function and arterial remodeling in PAH animals.
In conclusion, in this study, we showed that a novel combination therapy comprising PLGA particles of two distinct drugs, sildenafil and rosiglitazone, produced similar improvement in PAH pathology at a longer dosing interval compared with the combination of two plain drugs. The proposed therapy selectively reduced mPAP, improved cardiac function by increasing cardiac output and decreasing total pulmonary resistance, compensated for ventricular insufficiency by reducing RVH, and slowed disease progression by reducing collagen deposition and muscularization of the pulmonary arteries. Thus, this combination of inhaled long-acting particles of sildenafil plus rosiglitazone can potentially be translated into a clinically viable drug delivery system.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01-HL-114677 (to F. Ahsan), 1R35-HL-139726-01 (to E. Nozik-Grayck), and P01-HL-014985 and R01-HL-114887 and Department of Defense Grant PR140977 (to K. R. Stenmark).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R. and F.A. conceived and designed research; J.R. performed experiments; J.R. analyzed data; J.R. interpreted results of experiments; J.R. prepared figures; J.R. drafted manuscript; J.R., E.N.-G., I.F.M., K.R.S., and F.A. edited and revised manuscript; J.R., E.N.-G., and F.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical support from Crystal Woods at the University of Colorado Denver.
Present address of J. Rashid: 11404 Sorrento Valley Rd., San Diego, CA 92121.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 2.Alzoubi A, Toba M, Abe K, O’Neill KD, Rocic P, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 304: H1708–H1718, 2013. doi: 10.1152/ajpheart.00746.2012. [DOI] [PubMed] [Google Scholar]
- 3.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol 34: 1802–1806, 1999. doi: 10.1016/S0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 4.Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci 75: 433–438, 1986. doi: 10.1002/jps.2600750502. [DOI] [PubMed] [Google Scholar]
- 5.Chemla D, Castelain V, Hervé P, Lecarpentier Y, Brimioulle S. Haemodynamic evaluation of pulmonary hypertension. Eur Respir J 20: 1314–1331, 2002. doi: 10.1183/09031936.02.00068002. [DOI] [PubMed] [Google Scholar]
- 6.Colvin KL, Yeager ME. Animal models of pulmonary hypertension: matching disease mechanisms to etiology of the human disease. J Pulm Respir Med 4: 198, 2014. doi: 10.4172/2161-105X.1000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossno JT Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 8.Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci 55: 24–35, 2000. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- 9.EBSCO Health Pulmonary Arterial Hypertension (PAH) (Online) https://www.dynamed.com/topics/dmp~AN~T115043/Pulmonary-arterial-hypertension-PAH [6 November 2018].
- 10.Estrada KD, Chesler NC. Collagen-related gene and protein expression changes in the lung in response to chronic hypoxia. Biomech Model Mechanobiol 8: 263–272, 2009. doi: 10.1007/s10237-008-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox BD, Shtraichman O, Langleben D, Shimony A, Kramer MR. Combination therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Can J Cardiol 32: 1520–1530, 2016. doi: 10.1016/j.cjca.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 62, Suppl: D60–D72, 2013. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group . Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353: 2148–2157, 2005. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 14.Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARγ ligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. PLoS One 10: e0133391, 2015. doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta N, Patel B, Nahar K, Ahsan F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur J Pharm Biopharm 88: 1046–1055, 2014. doi: 10.1016/j.ejpb.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta N, Rashid J, Nozik-Grayck E, McMurtry IF, Stenmark KR, Ahsan F. Cocktail of superoxide dismutase and fasudil encapsulated in targeted liposomes slows PAH progression at a reduced dosing frequency. Mol Pharm 14: 830–841, 2017. doi: 10.1021/acs.molpharmaceut.6b01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta V, Ahsan F. Influence of PEI as a core modifying agent on PLGA microspheres of PGE1, a pulmonary selective vasodilator. Int J Pharm 413: 51–62, 2011. doi: 10.1016/j.ijpharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta V, Davis M, Hope-Weeks LJ, Ahsan F. PLGA microparticles encapsulating prostaglandin E1-hydroxypropyl-β-cyclodextrin (PGE1-HPβCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm Res 28: 1733–1749, 2011. doi: 10.1007/s11095-011-0409-6. [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Gupta N, Shaik IH, Mehvar R, McMurtry IF, Oka M, Nozik-Grayck E, Komatsu M, Ahsan F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J Control Release 167: 189–199, 2013. doi: 10.1016/j.jconrel.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta V, Gupta N, Shaik IH, Mehvar R, Nozik-Grayck E, McMurtry IF, Oka M, Komatsu M, Ahsan F. Inhaled PLGA particles of prostaglandin E1 ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency. Mol Pharm 10: 1655–1667, 2013. doi: 10.1021/mp300426u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansmann G, Zamanian RT. PPARγ activation: a potential treatment for pulmonary hypertension. Sci Transl Med 1: 12ps14, 2009. doi: 10.1126/scitranslmed.3000267. [DOI] [PubMed] [Google Scholar]
- 22.Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Sci 17: 811–825, 1986. doi: 10.1016/0021-8502(86)90035-2. [DOI] [Google Scholar]
- 23.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, Wilkins MR, Badesch DB. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D42–D50, 2013. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Huckaby J, Lawrence P, Center A, Simon D. Inhaled treprostinil via the Tyvaso Inhalation System through a tracheostomy. BMJ Case Rep 2015: bcr2015211602, 2015. doi: 10.1136/bcr-2015-211602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl S: S13–S24, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Jones R, Jacobson M, Steudel W. α-Smooth-muscle actin and microvascular precursor smooth-muscle cells in pulmonary hypertension. Am J Respir Cell Mol Biol 20: 582–594, 1999. doi: 10.1165/ajrcmb.20.4.3357. [DOI] [PubMed] [Google Scholar]
- 27.Joppi R, Gerardi C, Bertelé V, Garattini S. A disease looking for innovative drugs: the case of pulmonary arterial hypertension. Eur J Intern Med 55: 47–51, 2018. doi: 10.1016/j.ejim.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 15: 659–668, 2010. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 29.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 56: 588–599, 2003. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lajoie AC, Lauzière G, Lega JC, Lacasse Y, Martin S, Simard S, Bonnet S, Provencher S. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 4: 291–305, 2016. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 31.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 128: 1470–1479, 2013. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 32.Liu HL, Chen XY, Li JR, Su SW, Ding T, Shi CX, Jiang YF, Zhu ZN. Efficacy and safety of pulmonary arterial hypertension-specific therapy in pulmonary arterial hypertension: a meta-analysis of randomized controlled trials. Chest 150: 353–366, 2016. doi: 10.1016/j.chest.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchandise B, De Bruyne B, Delaunois L, Kremer R. Noninvasive prediction of pulmonary hypertension in chronic obstructive pulmonary disease by Doppler echocardiography. Chest 91: 361–365, 1987. doi: 10.1378/chest.91.3.361. [DOI] [PubMed] [Google Scholar]
- 35.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 114: 1417–1431, 2006. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin VV, Suissa S. Prognosis of pulmonary arterial hypertension: the power of clinical registries of rare diseases. Circulation 122: 106–108, 2010. doi: 10.1161/CIRCULATIONAHA.110.963983. [DOI] [PubMed] [Google Scholar]
- 38.Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev 23: 476–487, 2014. doi: 10.1183/09059180.00007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahar K, Absar S, Gupta N, Kotamraju VR, McMurtry IF, Oka M, Komatsu M, Nozik-Grayck E, Ahsan F. Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol Pharm 11: 4374–4384, 2014. doi: 10.1021/mp500456k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahar K, Rashid J, Absar S, Al-Saikhan FI, Ahsan F. Liposomal aerosols of nitric oxide (NO) donor as a long-acting substitute for the ultra-short-acting inhaled NO in the treatment of PAH. Pharm Res 33: 1696–1710, 2016. doi: 10.1007/s11095-016-1911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 43.Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC. The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1823–H1831, 2010. doi: 10.1152/ajpheart.00493.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel B, Gupta V, Ahsan F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release 162: 310–320, 2012. doi: 10.1016/j.jconrel.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989. doi: 10.1161/01.CIR.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 46.Rashid J, Alobaida A, Al-Hilal TA, Hammouda S, McMurtry IF, Nozik-Grayck E, Stenmark KR, Ahsan F. Repurposing rosiglitazone, a PPAR-γ agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH. J Control Release 280: 113–123, 2018. doi: 10.1016/j.jconrel.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rashid J, Nahar K, Raut S, Keshavarz A, Ahsan F. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery. Mol Pharm 15: 1755–1765, 2018. doi: 10.1021/acs.molpharmaceut.7b01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rashid J, Patel B, Nozik-Grayck E, McMurtry IF, Stenmark KR, Ahsan F. Inhaled sildenafil as an alternative to oral sildenafil in the treatment of pulmonary arterial hypertension (PAH). J Control Release 250: 96–106, 2017. doi: 10.1016/j.jconrel.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sardana M, Moll M, Farber HW. Novel investigational therapies for treating pulmonary arterial hypertension. Expert Opin Investig Drugs 24: 1571–1596, 2015. doi: 10.1517/13543784.2015.1098616. [DOI] [PubMed] [Google Scholar]
- 50.Shen JY, Chen SL, Wu YX, Tao RQ, Gu YY, Bao CD, Wang Q. Pulmonary hypertension in systemic lupus erythematosus. Rheumatol Int 18: 147–151, 1999. doi: 10.1007/s002960050074. [DOI] [PubMed] [Google Scholar]
- 51.Singh TP. Clinical use of sildenafil in pulmonary artery hypertension. Expert Rev Respir Med 4: 13–19, 2010. doi: 10.1586/ers.09.71. [DOI] [PubMed] [Google Scholar]
- 52.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 53.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 54.Sutliff RL, Kang BY, Hart CM. PPARγ as a potential therapeutic target in pulmonary hypertension. Ther Adv Respir Dis 4: 143–160, 2010. doi: 10.1177/1753465809369619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 56.Tissot C, Beghetti M. Review of inhaled iloprost for the control of pulmonary artery hypertension in children. Vasc Health Risk Manag 5: 325–331, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toba M, Alzoubi A, O’Neill K, Abe K, Urakami T, Komatsu M, Alvarez D, Järvinen TA, Mann D, Ruoslahti E, McMurtry IF, Oka M. A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension. Am J Pathol 184: 369–375, 2014. doi: 10.1016/j.ajpath.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tozzi CA, Christiansen DL, Poiani GJ, Riley DJ. Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am J Respir Crit Care Med 149: 1317–1326, 1994. doi: 10.1164/ajrccm.149.5.8173773. [DOI] [PubMed] [Google Scholar]
- 59.US Food and Drug Administration Avandia Prescribing Information. Silver Spring, MD: US Food and Drug Administration, 2014. [Google Scholar]
- 60.US Food and Drug Administration Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Silver Spring, MD: US Food and Drug Administration, 2005. [Google Scholar]
- 61.US Food and Drug Administration Revatio Prescribing Information (Online) https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021845lbl.pdf [6 November 2018].
- 62. US Food and Drug Administration FDA Drug Safety Communication: FDA Recommends Against Use of Revatio (Sildenafil) in Children with Pulmonary Hypertension. https://www.fda.gov/Drugs/DrugSafety/ucm317123.htm [31 January 2017].
- 63.US Food and Drug Administration Tyvaso Prescribing Information. (Online) https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022387LBL.pdf [6 November 2018].
- 64.US Food and Drug Administration Ventavis Prescribing Information (Online) https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021779lbl.pdf [6 November 2018].
- 65.Van Rheen Z, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E. Lung extracellular superoxide dismutase overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am J Respir Cell Mol Biol 44: 500–508, 2011. doi: 10.1165/rcmb.2010-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, El Kasmi KC, Savani RC, Bowler RP, Nozik-Grayck E. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal 18: 1753–1764, 2013. doi: 10.1089/ars.2012.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vonk-Noordegraaf A, Westerhof N. Describing right ventricular function. Eur Respir J 41: 1419–1423, 2013. doi: 10.1183/09031936.00160712. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Lakes RS, Eickhoff JC, Chesler NC. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech Model Mechanobiol 12: 1115–1125, 2013. doi: 10.1007/s10237-012-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williamson DJ, Wallman LL, Jones R, Keogh AM, Scroope F, Penny R, Weber C, Macdonald PS. Hemodynamic effects of bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation 102: 411–418, 2000. doi: 10.1161/01.CIR.102.4.411. [DOI] [PubMed] [Google Scholar]