Fig. 1.
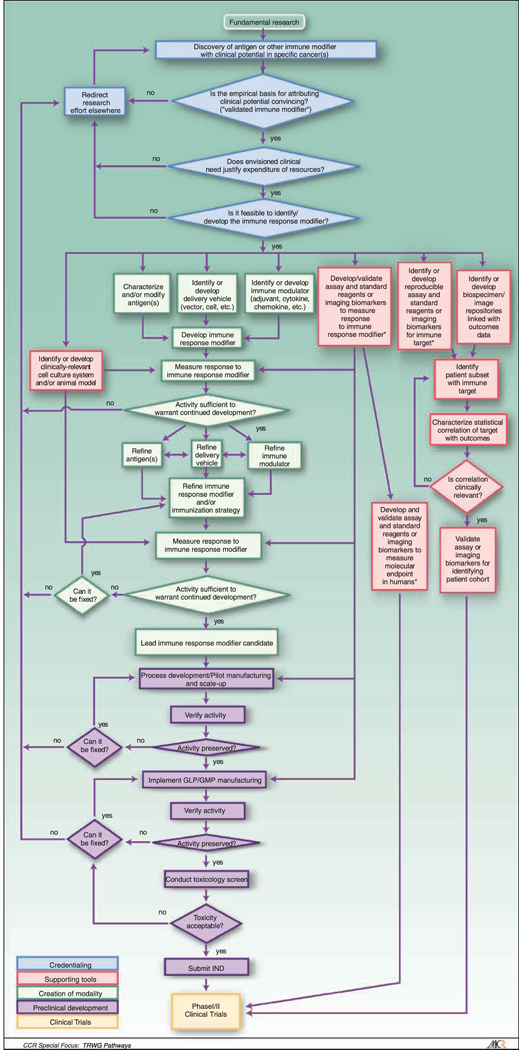
Immune Response Modifiers (IRM) Pathway. The IRM pathway is depicted as a flowchart, a schematic process representation widely used in engineering. Rounded rectangle at the top, origin of the process. Square-cornered rectangles, activity steps. Diamonds, conditional tests or decision steps. Unidirectional arrows, the direction of the activity sequence, and the direction of transfer of supporting tools from their parallel development paths to the main path of modality development. Bidirectional arrows, codevelopment or concurrent, interactive refinement. The initial steps of the pathway (blue) are required to proceed through the pathway, with the blue diamonds representing the credentialing steps of scientific validation, clinical need, and feasibility. The pathway proceeds to multiple parallel paths representing development of multiple components of the modality itself (green) as well as development of different classes of supporting tools (red). The three green boxes at the top of the creation of the modality path acknowledges the parallel and interactive development of a multicompent formulation and/or regimen, such as the need for an antigen, a delivery vehicle, and an immune modulator for the development of a cancer vaccine. The red boxes at the top of the supporting tools path depict the development of assays for characterizing and evaluating the effects of the modality and for defining the cohort for which the modality is appropriate. The development of these tools is further detailed in the Biospecimen-based or Imaging-based Assessment Modality pathway. Parallel paths have been made explicit to acknowledge that some of the required tools may not exist, and their parallel or codevelopment will be prerequisite for the viability of the new modality. Subsequent steps include preclinical development (purple) and early stage clinical trials (yellow). For each activity, decision point, parallel path, or feedback loop, it is understood that there are many more variations that can occur, and that not all steps may occur in each instance. The pathway does not address the ways in which insights gained from late-stage clinical trials can influence the development process. Immune response modifier agent interventions may be used for treatment or for primary, secondary, or tertiary prevention. The pathways are conceived not as comprehensive descriptions of the corresponding real-world processes but as tools designed to serve specific purposes, including research program and project management, coordination of research efforts, and professional and lay education and communication.
