Summary
Disrupted sleep is a contributing factor to cognitive ageing, while also being associated with neurodegenerative disorders. Little is known, however, about the relation of sleep and the gradual cognitive changes over the adult life course. Sleep electroencephalogram (EEG) patterns are potential markers of the cognitive progress. To test this hypothesis, we assessed sleep architecture and EEG of 167 men born in the Copenhagen Metropolitan Area in 1953, who, based on individual cognitive testing from early (~18 years) to late adulthood (~58 years), were divided into 85 subjects with negative and 82 with positive cognitive change over their adult life. Participants underwent standard polysomnography, including manual sleep scoring at age ~58 years. Features of sleep macrostructure were combined with a number of EEG features to distinguish between the two groups. EEG rhythmicity was assessed by spectral power analysis in frontal, central and occipital sites. Functional connectivity was measured by inter-hemispheric EEG coherence. Group differences were assessed by analysis of covariance (p < 0.05), including education and severity of depression as potential covariates. Subjects with cognitive decline exhibited lower sleep efficiency, reduced inter-hemispheric connectivity during rapid eye movement (REM) sleep, and slower EEG rhythms during stage 2 non-REM sleep. Individually, none of these tendencies remained significant after multiple test correction; however, by combining them in a machine learning approach, the groups were separated with 72% accuracy (75% sensitivity, 67% specificity). Ongoing medical screenings are required to confirm the potential of sleep efficiency and sleep EEG patterns as signs of individual cognitive progress.
Keywords: cognitive ageing, electroencephalogram, functional connectivity, neurodegeneration, sleep efficiency
1. Introduction
Sleep has a vital role in a range of physiological functions, from tissue re-saturation to brain-metabolite clearance, and memory stabilization and integration. With increasing age, there is a change in both sleep quality and quantity. The sleep cycles become more fragmented and more time is spent in "lighter" sleep stages. Insomnia and excessive daytime sleepiness are frequently reported (Scullin & Bliwise, 2015). Although retained slow wave sleep at a high age has been associated with better cognitive functioning (Anderson & Horne, 2003), sleep disruptions in the elderly represent a contributing factor to cognitive ageing (Mander et al., 2013). Severe forms of sleep impairment have also been associated with major neurodegenerative disorders (Porter, Buxton, & Avidan, 2015). Patients with Alzheimer's disease often exhibit accelerated sleep fragmentation together with a reduction of rapid eye-movement (REM) and deep sleep (Avidan, 2006; Dowling et al., 2008). Frontotemporal dementia is associated with increased frequency of insomnia (Anderson, Hatfield, Kipps, Hastings, & Hodges, 2009). Up to 90% of patients diagnosed with Parkinson's disease or dementia with Lewy bodies suffer from sleep disturbances such as insomnia and REM behavioural disorder (RBD) (Kumar, Bhatia, & Behari, 2002). Vascular dementia is commonly accompanied by sleep-disordered breathing and severe daytime sleepiness, both worsening with disease progression (Porter et al., 2015). Moreover, pharmacological interventions used for neurological conditions (e.g. antiepileptic drugs) frequently affect sleep routines by inducing daytime sleepiness, disrupted sleep cycles or even RBD (Trotti, 2010). As yet, however, the connection between sleep and cognitive function has not been fully understood (Yaffe, Falvey, & Hoang, 2014).
Along with the macro-changes in sleep architecture, sleep-specific electroencephalography (EEG) alterations have been reported for several neurodegenerative disorders, with slowing of EEG rhythms being the most observed phenomenon (Petit, Gagnon, Fantini, Ferini-Strambi, & Montplaisir, 2004). EEG slowing describes a shift from fast EEG oscillations in the alpha, beta and gamma frequency range above ~8 Hz to slow wave, delta and theta ranges. This shift is commonly quantified by changes in spectral EEG power in the aforementioned frequency bands. In Alzheimer's disease, REM sleep is particularly affected by EEG slowing, which was found to be more marked during this stage than during wakefulness (Petit, Montplaisir, Lorrain, & Gauthier, 1992). A possible explanation is that EEG activity during REM sleep is mainly the result of neurons in the cholinergic nucleus basalis and the glutamatergic thalamocortical system, which are typically affected by cholinergic deficits in Alzheimer's disease (Petit et al., 2004). Patients with RBD exhibited reduced EEG beta power, possibly reflecting early central nervous system dysfunction (Fantini et al., 2003). The defining EEG characteristics of healthy stage 2 non-REM (NREM) sleep are sleep spindles and K-complexes. They are associated with memory consolidation and become less numerous in progressive supranuclear palsy, Creutzfeldt-Jakob disease and Alzheimer's disease, the latter also affecting their amplitude and duration (Petit et al., 2004). Alterations in sleep spindle density have been observed in patients with Huntington's disease (Wiegand et al., 1991). Petit et al. (2004) conclude that studying "the spectral composition of the EEG in different states can provide valuable information for understanding the pathophysiology and assisting the diagnosis of neurodegenerative diseases."
The clinical diagnosis of a neurodegenerative disorder is often preceded by a long stage of cognitive decline, including subjective memory complaints. Although the gradual decline in cognitive function has been the subject of various studies, only a few of them consider the gradual changes in sleep patterns during this stage as well. Nevertheless, sleep disruptions in a premorbid stage may be a strong indicator of the later development of neurodegenerative disorders. Based on this assumption, we hypothesized that sleep structure and neurophysiology in midlife are affected in subjects with different cognitive progression over their adult life course. To test this hypothesis, we compared both the sleep architecture and sleep EEG of male individuals with negative cognitive changes (N = 85) and with positive cognitive changes (N = 82) as determined from longitudinal cognitive testing from adolescence to late adulthood (from age 18 to 56 years). This study provides new insights into the potential of sleep and sleep EEG as markers of cognitive changes over the adult age span.
2. Materials and Methods
2.1. Participants
This study included 167 male participants, who are a subsample of the Metropolit Danish Male Birth (Osler, Lund, Kriegbaum, Christensen, & Andersen, 2006) cohort and the Copenhagen Aging and Midlife Biobank (CAMB; Avlund et al., 2014) study. The Metropolit cohort consisted of all men born in 1953 in the Copenhagen metropolitan area (n = 11,532) and contains information on their health and sociodemographic factors. Some 1,985 participants were cognitively assessed using the Børge Priens Prøve (BPP; Teasdale, 2009) test at their draft board examination at age ~18 years, and again at age ~58 years using the Intelligenz-Struktur-Test 2000 R (I-S-T 2000 R; Amthauer, 2001) as part of the CAMB study. BPP and I-S-T 2000 R comprise similar subtests of verbal and numerical reasoning (Osler, Avlund, & Mortensen, 2013). On the basis of linear regression analysis with BPP score as predictor and I-S-T 2000R score as outcome, the study of ageing-affected cognition at the Center for Healthy Aging (University of Copenhagen) selected 552 subjects divided into two groups (Hansen et al., 2014): subjects with large positive residuals (i.e. cognitive improvement) and subjects with large negative residuals (i.e. cognitive decline). The two groups did not differ with respect to their baseline cognitive function. The level of completed formal education was introduced as a potential covariate into our analysis (cf. Waller et al., 2016). Subjects were excluded if they had medical conditions with a well-established association with cognitive dysfunction, such as alcohol and drug abuse, major psychiatric and neurologic disease, and brain lesions. Depressive symptoms were assessed using the Beck's Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996) and introduced as potential covariates as well. Between 2009 and 2013, a subsample of 207 subjects underwent detailed clinical evaluations, including sleep monitoring by standard polysomnography (PSG) at the Danish Center for Sleep Medicine (Rigshospitalet Glostrup). Manual sleep scoring has been completed for 167 subjects (82 with cognitive improvement, 85 with cognitive decline), who represent our study sample.
2.2. Ethics statement
The study was approved by the regional ethics committee (Scientific Ethics Committee of the Capital Region, Protocol no. H-3-2010-016) following the standards of the National Committee of Health Research Ethics. The examinations were conducted in accordance with the Helsinki Declaration and all men gave written consent.
2.3. Sleep monitoring
Sleep electroencephalogram (EEG) signals were collected in a contralateral ear reference montage from six scalp electrodes (F3, F4, C3, C4, O1 and O2) placed according to the international 10–20 system (Jasper, 1958). EEG electrodes were re-referenced to the mathematically linked ear lobes. The sampling rate was 256 Hz with a resolution of 16 bits. Impedances were kept below 5 kΩ. Additional recordings included electrooculogram (EOG; right and left outer canthi), electrocardiogram (ECG) and electromyogram (EMG; chin, right and left anterior tibialis), as well as respiration (nasal airflow, thoracic and abdominal movement), oxygen saturation and snoring. The sleep recordings were manually scored by a blinded clinical expert on a 30-s basis in accordance with the standard AASM criteria (Iber, Ancioli-Israel, Chesson, & Quan, 2007).
2.4. Sleep features
We computed features of sleep macrostructure, EEG rhythmicity and functional connectivity (see Table 1).
Table 1.
Features of sleep macrostructure, EEG rhythmicity and functional EEG connectivity
| Feature | Definition |
|---|---|
| Total sleep duration (min) | Time in bed spent in REM or NREM stage |
| Total stage duration (min) | Time in bed spent in each stage |
| Relative stage duration (%) | Percentage of sleep (from first non-W to last non-W) spent in each stage |
| Number of stage episodes | Number of stable (minimum 3 min) episodes in each stage |
| Mean stage duration (min) | Mean duration of episodes in each stage |
| Sleep latency (min) | Time in bed after lights are off to reach the first non-W stage |
| REM sleep latency (min) | Time in bed after first non-W stage to reach REM sleep |
| Sleep efficiency (%) | Percentage of time in bed after lights are off spent in a non-W stage |
| EEG slow wave power | Spectral EEG power in the frequency range 0.5–1.5 Hz |
| EEG delta power | Spectral EEG power in the frequency range 1.5–4 Hz |
| EEG theta power | Spectral EEG power in the frequency range 4–7.5 Hz |
| EEG alpha power | Spectral EEG power in the frequency range 7.5–11 Hz |
| EEG sigma power | Spectral EEG power in the frequency range 11–15 Hz |
| EEG beta power | Spectral EEG power in the frequency range 15–30 Hz |
| EEG slow wave coherence | Inter-hemispheric coherence in the frequency range 0.5–1.5 Hz |
| EEG delta coherence | Inter-hemispheric coherence in the frequency range 1.5–4 Hz |
| EEG theta coherence | Inter-hemispheric coherence in the frequency range 4–7.5 Hz |
| EEG alpha coherence | Inter-hemispheric coherence in the frequency range 7.5–11 Hz |
| EEG sigma coherence | Inter-hemispheric coherence in the frequency range 11–15 Hz |
| EEG beta coherence | Inter-hemispheric coherence in the frequency range 15–30 Hz |
EEG, electroencephalogram; REM, rapid eye movement; NREM, non-rapid eye movement; W, wake.
2.4.1. Sleep macrostructure
The sleep macrostructure is the basic structural organization of sleep that consists of cyclically distributed REM and NREM sleep, as well as episodes of wakefulness (W). NREM is commonly divided into N1, N2 and N3 sleep. Short or disrupted sleep cycles are indicators of a high degree of sleep fragmentation. To quantify different sleep aspects, we employed a set of features: the total duration of sleep and of the individual sleep stages, the percentage spent in each stage, the number of episodes in each stage, and the mean episode duration of each stage. A minimum of 3 uninterrupted minutes in a stage was considered a stable episode. Additional features concerning the sleep quality comprised sleep efficiency, sleep latency and REM sleep latency.
2.4.2. EEG rhythmicity and functional connectivity
The first 3 consecutive minutes in each sleep stage were selected and quantitative EEG analysis was performed for the central 2 min to avoid sleep stage transition. EEG processing was carried out using MATLAB software (MathWorks, Natick, MA, USA). The signals were band-pass filtered in the range of 0.5–30 Hz (type 1 finite impulse response filter, 60 dB). Artifacts from eye movement, cardiac activity or muscular activity were corrected using independent component analysis incorporating the EOG, ECG and EMG channels (Lu & Rajapakse, 2006). Residual artifacts were removed on a 3-s basis by visual inspection of all recorded channels and omitted from further analysis. In the presence of electrode failure or continuous excessive artifacts in individual electrodes, these channels were excluded and treated as missing data (n = 16 or 1.6% of all channels). Spectral EEG analysis was performed by indirect spectral estimation using a 3-s Parzen window with a 2-s overlap and subsequent averaging of the power spectral densities from all respective 3-s mini-epochs of an individual sleep stage (Brillinger, 1981). The spectral EEG band-power as measure of rhythmicity was computed by bin-wise power summation in the following frequency ranges: slow waves (0.5–1.5 Hz), delta (1.5–4 Hz), theta (4–7.5 Hz), alpha (7.5–11 Hz), sigma (11–15 Hz) and beta (15–30 Hz). As a measure of functional connectivity, we computed the inter-hemispheric coherence in the same frequency bands for F3-F4, C3-C4 and O1-O2.
2.5. Statistical analysis
Group differences in severity of depression and level of education were assessed by the Mann–Whitney U-test. The (continuous) features were normalized to z-scores and their group-wise normality was tested by Kolmogorov–Smirnov tests. The assumption of group variance equality was tested by Levene's test. Group differences of each feature were then evaluated by analyses of covariance (ANCOVA) including potential covariate(s). Results were considered significant for p < 0.05. In total, our analysis included 78 statistical tests. Multiple comparison correction was performed by the Holm–Bonferroni method (Holm, 1979). Significant features were then used as the input of a support vector machine (SVM) classifier with radial basis function and subsequent receiver operating characteristic (ROC) curve analysis. The classification performance was evaluated by the area under the ROC curve (AUC), sensitivity, specificity and accuracy, all estimated using five-fold cross-validation to avoid overfitting to our sample. In this approach, the cohort was randomly partitioned into five equal-sized subsamples, of which a single subsample was used as validation data and the remaining four subsamples as training data for the SVM model. This procedure was repeated five times, with each of the five subsamples used once as validation data. The five results from the folds were then averaged to produce a single estimate of the classification performance.
3. Results
3.1. Sample characteristics
Our sample included 167 male participants born in the Copenhagen metropolitan area in 1953. Based on longitudinal cognitive assessments at age ~18 years and again at ~58 years, the sample was divided into 82 subjects with cognitive improvement and 85 with cognitive decline over their adult life course. The two groups did not differ with respect to their baseline cognitive function. Four subjects had mild (BDI-II score between 14 and 19) and one subject had severe (BDI-II greater than 28) depressive symptoms; the BDI-II test was not completed in four cases and the respective scores were treated as missing values. No significant group differences were found between cognitively improved and impaired subjects (p = 0.341). The cognitively impaired group, however, had less formal education than the cognitively improved group (p < 0.001). Education was therefore included as a covariate in the further analyses.
3.2. Sleep macrostructure
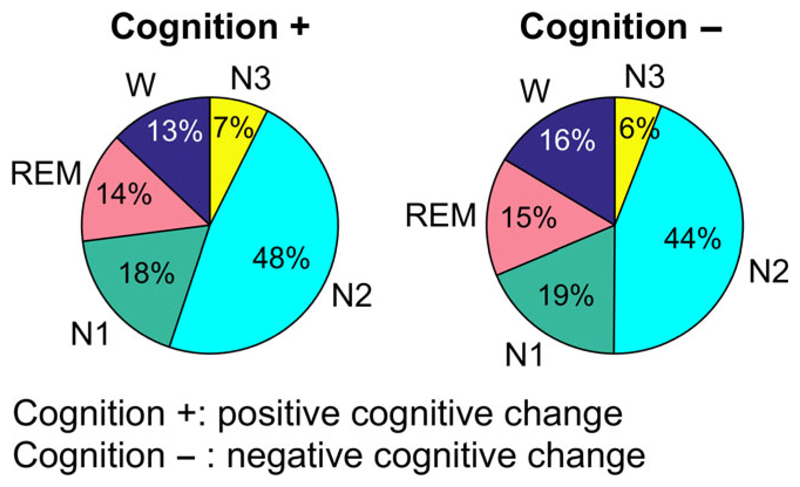
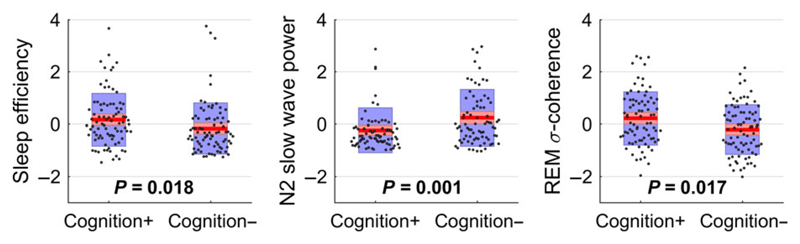
Figure 1 shows the average sleep stage distribution by group (positive and negative cognitive change) as percentage values. Although there were no or only small group differences regarding REM, N1 and N3 sleep, the subjects with cognitive decline exhibited reduced N2 sleep and prolonged wakefulness during the night. This was mainly caused by a prolonged average wake episode duration and not by an increased number of W episodes. The prolonged time spent awake during the night resulted in a decrease of sleep efficiency that did not remain significant after multiple test correction. Figure 3 (left) shows a boxplot of the sleep efficiency (as z-score values), which was significantly reduced in the cognitively impaired group (p = 0.018) prior to multiple test correction. Both the total time spent in N2 sleep and the number and mean duration of N2 episodes were non-significant. Table 2 lists the significant (prior to multiple test correction) features of sleep efficiency with their group-wise mean and standard deviation, as well as the p-values of ANCOVA testing and the corresponding Bonferroni–Holm-corrected alpha levels. The complete list of features is provided in Supporting Information, Table S1.
Figure 1.
Average sleep-stage distribution of subjects with positive cognitive change (n =82) and with negative cognitive change (n = 85) over their adult life course. The latter spend more time during the night awake and less time in N2 sleep
Figure 3.
Boxplots of three selected features (z-score values) including p-values of ANCOVA with education as a covariate. Sleep efficiency, slow wave power at O1 during N2 and sigma coherence at C3-C4 during REM were (prior to multiple comparison correction) significantly different between the groups. After Bonferroni–Holm correction, these tendencies were non-significant and should therefore not be considered as stand-alone features but combined in a multivariate approach. Cognition +, positive cognitive change; Cognition −, negative cognitive change
Table 2.
List of significant features (prior to multiple comparison correction) reflecting group tendencies in sleep efficiency, slow EEG activity in N2, and inter-hemispheric EEG coherence in REM sleep between subjects with positive (n = 82) and with negative cognitive change (n = 85). Because none of the tendencies remained significant after Bonferroni–Holm correction, they should not be considered as stand-alone features but combined in a multivariate approach
| Feature | Cognition + Mean ± SD | Cognition − Mean ± SD | p | Corrected α-level | |
|---|---|---|---|---|---|
| Sleep macrostructure | |||||
| Total W duration (min) | 55.1 ± 49.8 | 69.0 ± 45.0 | 0.026 | 0.00072 | |
| Relative W duration (%) | 12.9 ± 10.6 | 16.4 ± 10.6 | 0.016 | 0.00066 | |
| Relative N2 duration (%) | 47.7 ± 11.3 | 44.2 ± 12.1 | 0.028 | 0.00075 | |
| Sleep efficiency (%) | 84.4 ± 10.9 | 80.7 ± 11.2 | 0.018 | 0.00068 | |
| Spectral EEG power during N2 sleep | |||||
| Slow waves (0.5–1.5 Hz) | at F3 | 93.5 ± 70.7 | 117.3 ± 87.4 | 0.022 | 0.00070 |
| Slow waves (0.5–1.5 Hz) | at F4 | 99.1 ± 76.4 | 121.4 ± 88.2 | 0.028 | 0.00074 |
| Slow waves (0.5–1.5 Hz) | at C3 | 64.1 ± 48.5 | 74.9 ± 47.2 | 0.044 | 0.00079 |
| Slow waves (0.5–1.5 Hz) | at C4 | 64.0 ± 45.5 | 77.9 ± 46.0 | 0.016 | 0.00065 |
| Slow waves (0.5–1.5 Hz) | at O1 | 17.2 ± 15.3 | 25.8 ± 19.4 | 0.001 | 0.00064 |
| Slow waves (0.5–1.5 Hz) | at O2 | 21.0 ± 21.2 | 26.2 ± 19.2 | 0.047 | 0.00081 |
| Delta (1.5–4 Hz) | at O1 | 28.3 ± 26.3 | 36.2 ± 23.1 | 0.024 | 0.00071 |
| EEG coherence during REM sleep | |||||
| Delta (1.5–4 Hz) | at C3-C4 | 0.82 ± 0.09 | 0.78 ± 0.11 | 0.021 | 0.00069 |
| Delta (1.5–4 Hz) | at O1-O2 | 0.67 ± 0.13 | 0.62 ± 0.17 | 0.017 | 0.00068 |
| Theta (4–7.5 Hz) | at C3-C4 | 0.75 ± 0.12 | 0.71 ± 0.12 | 0.032 | 0.00077 |
| Alpha (7.5–11 Hz) | at C3-C4 | 0.66 ± 0.14 | 0.61 ± 0.14 | 0.030 | 0.00076 |
| Sigma (11–15 Hz) | at C3-C4 | 0.63 ± 0.15 | 0.56 ± 0.14 | 0.017 | 0.00067 |
| Beta (15–30 Hz) | at C3-C4 | 0.55 ± 0.16 | 0.50 ± 0.13 | 0.034 | 0.00078 |
Notes. EEG, electroencephalogram; REM, rapid eye movement; Cognition +, positive cognitive change; Cognition −, negative cognitive change; SD, standard deviation; W, wake.
p: p-values of ANCOVA including level of education as covariate.
Corrected α-level: Bonferroni–Holm-corrected significance levels.
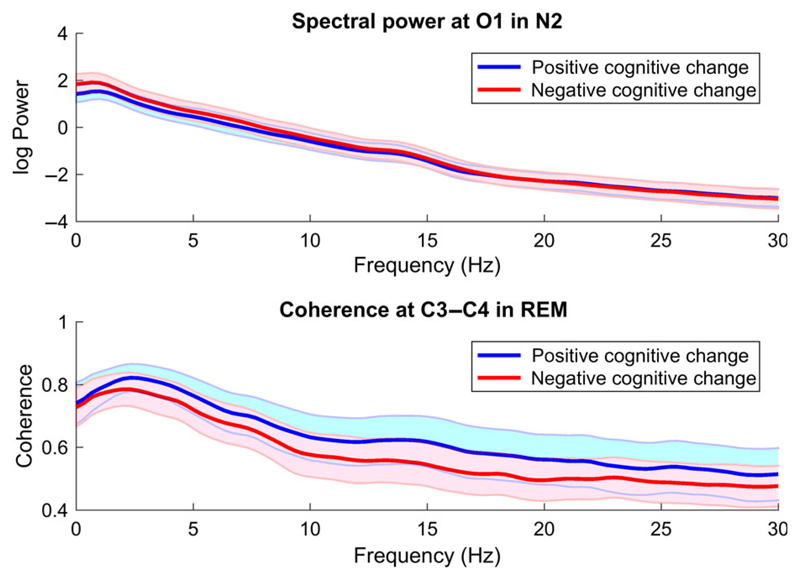
3.3. EEG rhythmicity
Visual inspection of the sleep EEG spectral density indicated an increased absolute power in a low-frequency range below 8 Hz during N2 sleep in subjects with negative cognitive change as compared with those with cognitive improvement. Figure 2 (top) shows the EEG slowing by the group-wise average absolute spectral power on O1 during N2 sleep. Group-difference testing resulted in significantly increased slow wave, delta and theta power in cognitively declined subjects at most electrode sites, most prominently in the slow wave range below 1.5 Hz; however, none of these group differences survived post-correction. Figure 3 (middle) shows a boxplot of the slow wave power at O1 during N2 (as z-score values) including the ANCOVA p-value of 0.001. The second part of Table 2 lists the significant features of EEG slowing in N2 sleep with their group-wise mean and standard deviation values, as well as the ANCOVA p-values and the post-corrected significance levels. The complete list of features in N2 is provided in Supporting Information, Table S2.
Figure 2.
Group-wise mean (thick lines) and standard deviation (shaded areas) of absolute spectral power in O1 during N2 sleep (upper panel) and of coherence in C3-C4 during REM sleep (lower panel). Subjects with negative cognitive change exhibit higher low-frequency power ("slower" EEG) and lower inter-hemispheric coherence (functional disconnection)
3.4. Functional EEG connectivity
An initial visual coherence assessment suggested group differences during REM sleep, mainly localized in the centrally positioned interhemispheric channels. Figure 2 (bottom) shows the group-wise average coherence values on the channel-pair C3-C4. With the exception of the slow wave frequency range, subjects with negative cognitive change exhibited an evident reduction in coherence. This observation was affirmed by ANCOVA testing but did not survive multiple test correction. Figure 3 (right) shows a boxplot of the sigma coherence at C3-C4 during REM (as z-score values) including the ANCOVA p-value of 0.017. The third part of Table 2 contains the significant coherence values in C3-C4 in REM sleep, together with the group-wise mean and standard deviation values, as well as the corresponding p-values and corrected alpha levels. The complete list of group-wise coherences in REM is provided in Supporting Information, Table S3. No differences in coherence were observed for the remaining sleep stages.
3.5. Combined features
The analysis of sleep macrostructure, EEG rhythmicity and functional EEG connectivity indicated group trends that could not be confirmed as stand-alone markers after post-correction. By combining the features, we attempted to overcome this limitation. Based on the previous analyses, we observed three main tendencies in the cognitively impaired group as compared with the cognitively improved one: (a) reduced sleep efficiency, (b) slower EEG activity in N2 and (c) reduced inter-hemispheric coherence during REM sleep. The corresponding features were used as the input for an SVM classifier with five-fold cross-validation. Table 3 includes the performance metrics (AUC, accuracy, sensitivity, specificity) of each fold, as well as each average value. The average AUC value was 0.69 where 0.50 would be a random result and 1.00 would be an optimum classification. The two groups were separated with an average accuracy of 72% (sensitivity 75%, specificity 67%).
Table 3.
Classification performance: the five-fold cross-validation resulted in an average classification accuracy of 72%
| AUC | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|
| Fold 1 | 0.69 | 0.69 | 0.60 | 0.79 |
| Fold 2 | 0.71 | 0.72 | 0.75 | 0.71 |
| Fold 3 | 0.68 | 0.71 | 0.94 | 0.42 |
| Fold 4 | 0.54 | 0.64 | 0.62 | 0.67 |
| Fold 5 | 0.85 | 0.82 | 0.86 | 0.79 |
| Average | 0.69 | 0.72 | 0.75 | 0.67 |
4. Discussion
In this study, we hypothesized that sleep structure and neurophysiology in midlife were affected in male individuals with different cognitive progression over their adult life course. The homogeneous study sample of 167 middle-aged Danish men showed three potential indicators of differences in cognitive progression. Although, individually, none of these indicators was significant after multiple test correction, their combination discriminated positive and negative cognitive progression with an accuracy of 72%; therefore, these effects might still contain relevant diagnostic information. The first observation was a reduced sleep efficiency in subjects with negatively changed cognition as a result of prolonged (but equal number of) episodes of wakefulness. Although nightly awakenings are a common phenomenon during ageing, this effect seems to be accelerated in subjects with individual cognitive decline. A previous study in the same cohort reported an association of cognitive decline and poor self-rated sleep quality (Waller et al., 2016). Low sleep efficiency is evidently connected with subjective low sleep quality; the parameters might thus represent two sides of the same coin. Because sleep is supposedly essential for memory and cognition, an intriguing question is whether the reduced sleep efficiency represents a mere side-effect of impaired cognitive ageing or is a contributing factor to the cognitive decline (Mander et al., 2013). If the latter is true, the natural follow-up question is whether improving sleep quality has the potential to decelerate or even reverse the cognitive decline (Scullin & Bliwise, 2015). This so-called sleep-cognition hypothesis (Feinberg & Evarts, 1969) has been widely discussed but as of yet no definite answer has been found.
The second observation was a tendentially slower EEG rhythm as measured by increased slow wave, delta and theta power in subjects with cognitive decline. Slowing of wakefulness EEG is a common phenomenon in a range of neurological disorders, including Alzheimer's disease, but has also been described in mild cognitive impairment (Jeong, 2004). Particularly, theta rhythms have been associated with cognitive performance, because theta synchronization showed strong correlations with working memory and episodic memory performance (Klimesch, 1999). EEG slowing has also been described during REM sleep of Alzheimer's patients (Hassania, Petit, Nielsen, Gauthier, & Montplaisir, 1997). Petit et al. (2004) speculated that the consequences of cholinergic deficits for cortical activation may be more pronounced in REM sleep because cortical activation during this stage is mainly the result of the cholinergic system. Here, we found EEG slowing during N2 sleep, which is in line with a reported increase in NREM delta power in demented subjects (Bonanni et al., 2012). A recent study, on the other hand, found decreased NREM EEG delta power in a group of highly intelligent individuals as compared with those with average intelligence, suggesting that sleep EEG delta power reflects neural plasticity and cortical homeostatic processes (Pótári et al., 2017). De Gennaro et al. (2017) explained this apparent contradiction by the partial frequency overlap between delta EEG activity and K-complexes that have been associated with memory consolidation. The K-complex number and shape are altered in the course of neurodegeneration but seem to be unaltered in earlier stages of cognitive impairment (Reda et al., 2017). The other N2-defining EEG events, sleep spindles, are a product of thalamocortical innervation and mainly occur in the sigma frequency range between 11 and 15 Hz. We found no group effects regarding sigma power, a potential indicator that the corresponding neural system is not substantially associated with cognitive progress at this stage.
Finally, the study participants with cognitive decline tended to have reduced inter-hemispheric EEG coherence during REM sleep. Coherence has been widely used as a non-invasive measure of functional connectivity between cortical areas. A related study found a correlation of steady-state gamma coherence evoked by different stimulation types with long-term memory capacity (Horwitz et al., 2017). Reduced EEG coherence is also a well-established feature in Alzheimer's disease with correlation with the degree of cognitive impairment. Besides the anatomical loss of cortico–cortical association fibres, Jeong (2004) described reduced coherence as an indicator of functional changes in synaptic couplings among cortical networks as a result of reduced cholinergic coupling interactions. The functional nature of these changes offers a potential explanation for the fact that the coherence was not equally affected in all frequency bands (Besthorn et al., 1994; Stam, van der Made, Pijnenburg, & Scheltens, 2003). Here, the decreased coherence was evident mostly for the central inter-hemispheric sites C3 and C4. A topographic explanation might be the cholinergic activation of the cortical areas beneath the electrodes (i.e. somatosensory and motor cortices) during REM sleep (De Carli et al., 2016). However, the reason could also be a mere technical one: the central recording sites are commonly unaffected by most artifacts as compared with frontal and occipital sites.
This study offers important insights into the potential of sleep and sleep EEG as markers of cognitive changes over the adult age span. The cohort consisted of 167 middle-aged Danish men born in the same area in the same year; thus there was no need for correction for age, sex and cultural background. However, a larger and more heterogeneous cohort is needed to further investigate potential sleep-related markers of cognitive progression. We considered depressive symptoms and the completed level of formal education as potential covariates; although there was no group difference regarding depression, the participants' education was introduced as a covariate. This is well in accordance with a previous related study that has considered the influence of potential covariates associated with cognitive decline (Rask et al., 2016). Another critical point is the potential influence of pathological EEG patterns on the manual sleep stage scoring. The absence of sleep spindles and K-complexes because of a neurological condition, for example, complicates the staging of N2 sleep, which in turn increases inter-scorer variability and introduces a potential bias to studies like ours (Iber et al., 2007). Here, we tried to overcome this limitation by including only "stable" sleep stages and avoiding epochs of stage transition.
5. Conclusion
In conclusion, the study design involving cognitive testing from early (~18 years) to late adulthood (~58 years) enabled the analysis of sleep in 167 male subjects with positive and negative individual cognitive changes over their adult age span. Our results suggest an association of cognitive decline with reduced sleep efficiency, EEG slowing in N2 sleep and reduced inter-hemispheric EEG coherence in REM sleep. Although none of these measures passed as standalone features, the combined effects discriminated the two groups with an accuracy of 72% (sensitivity 75%, specificity 67%), thereby showing the potential of combined sleep and electrophysiological measures as signs of cognitive changes. Larger and more heterogeneous populations are needed for a proper validation of our observations. Most relevant, however, will be the analysis of medical screenings of the cohort in the coming years to identify subjects progressing from cognitive decline to severe neurological disorders. These future investigations will need to confirm the demonstrated potential of sleep disruptions as strong indicators of a possible later development of a neurodegenerative disorder.
Supplementary Material
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Acknowledgements
This research was funded by the Austrian Science Fund (FWF): project number J3766. The neurophysiological examination was funded by the Nordea Foundation (Center for Healthy Ageing, University of Copenhagen). The Copenhagen Aging and Midlife Biobank (CAMB) was funded by the Velux foundation. We thank all those who initiated and/or continued the Metropolit study: K Svalastoga, E Høgh, P Wolf, T Rishøj, G Strande-Sørensen, E Manniche, B Holten, IA Weibull and A Ortman. The authors also thank H Bruunsgaard, N-E Fiehn, ǺM Hansen, P Holm-Pedersen and R Lund, who initiated and established CAMB from 2009 to 2011, together with K Avlund, EL Mortensen and M Osler.
Funding information
Austrian Science Fund, Grant/Award Number: J3766; Velux Fonden; Nordea-fonden
Footnotes
Conflicts of Interest
None of the authors report any conflicts of interest.
Author Contribution
M Waser: study conception/design, statistical analysis and data interpretation, and drafting of manuscript. MJ Lauritzen: study conception/design, acquisition of data and critical revision of the manuscript. B Fagerlund: study conception/design, acquisition of data and critical revision of the manuscript. M Osler: study conception/design, acquisition of data and critical revision of the manuscript. EL Mortensen: study conception/design, acquisition of data and critical revision of the manuscript. HBD Sorensen: study conception/design and critical revision of the manuscript. P Jennum: study conception/design, acquisition of data and critical revision of the manuscript.
ORCID
Markus Waser https://orcid.org/0000-0002-8964-4675
References
- Amthauer R. Intelligenz-Struktur-Test 2000 R: I-S-T 2000 R Manual. 2 Auflage. Göttingen: Hogrefe, Verl. für Psychologie; 2001. [Google Scholar]
- Anderson KN, Hatfield C, Kipps C, Hastings M, Hodges JR. Disrupted sleep and circadian patterns in frontotemporal dementia. European Journal of Neurology. 2009;16:317–323. doi: 10.1111/j.1468-1331.2008.02414.x. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: Links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Avidan AY. Sleep and neurologic problems in the elderly. Sleep Medicine Clinics. 2006;1:273–292. doi: 10.1016/j.jsmc.2006.04.010. [DOI] [Google Scholar]
- Avlund K, Osler M, Mortensen EL, Christensen U, Bruunsgaard H, Holm-Pedersen P, et al. Lund R. Copenhagen Aging and Midlife Biobank (CAMB): An introduction. Journal of Aging and Health. 2014;26(1):5–20. doi: 10.1177/0898264313509277. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Besthorn C, Forstl H, Geiger-Kabisch C, Sattel H, Gasser T, Schreiter-Gasser U. EEG coherence in Alzheimer disease. Electroencephalography and Clinical Neurophysiology. 1994;90:242–245. doi: 10.1016/0013-4694(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonanni E, Di Coscio E, Maestri M, Carnicelli L, Tsekou H, Economou NT, et al. Ktonas PY. Differences in EEG delta frequency characteristics and patterns in slow-wave-sleep between dementia patients and controls: A pilot study. Journal of Clinical Neurophysiology. 2012;29:50–54. doi: 10.1097/wnp.0b013e318246b56d. [DOI] [PubMed] [Google Scholar]
- Brillinger DR. Time series: Data analysis and theory. San Francisco: Holden-Day; 1981. [Google Scholar]
- De Carli F, Proserpio P, Morrone E, Sartori I, Ferrara M, Gibbs SA, et al. Nobili L. Activation of the motor cortex during phasic rapid eye movement sleep. Annals of Neurology. 2016;79:326–330. doi: 10.1002/ana.24556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Gorgoni M, Reda F, Lauri G, Truglia I, Cordone S, et al. Rossini PM. The fall of sleep K-complex in Alzheimer disease. Scientific Reports. 2017;7 doi: 10.1038/srep39688. 39688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. Journal of the American Geriatrics Society. 2008;56:239–246. doi: 10.1111/(issn)1532-5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini ML, Gagnon J-F, Petit D, Rompré S, Décary A, Carrier J, Montplaisir J. Slowing of EEG in idiopathic REM sleep behavior disorder. Annals of Neurology. 2003;53:774–780. doi: 10.1002/ana.10547. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Evarts EV. Changing concepts of the function of sleep: Discovery of intense brain activity during sleep calls for revision of hypotheses as to its function. Biological Psychiatry. 1969;1:331–348. [PubMed] [Google Scholar]
- Hansen NL, Lauritzen M, Mortensen EL, Osler M, Avlund K, Fagerlund B, Rostrup E. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain's default mode network. Human Brain Mapping. 2014;35:4488–4498. doi: 10.1002/hbm.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassania F, Petit D, Nielsen T, Gauthier S, Montplaisir J. Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer's disease. European Neurology. 1997;37:219–224. doi: 10.1159/000117446. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Horwitz A, Mortensen EL, Osler M, Fagerlund B, Lauritzen M, Benedek K. Passive double-sensory evoked coherence correlates with long-term memory capacity. Frontiers in Human Neuroscience. 2017;11:598. doi: 10.3389/fnhum.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancioli-Israel S, Chesson AL, Quan SF, for the American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer's disease. Clinical Neurophysiology. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson's Disease. Movement Disorders. 2002;17:775–781. doi: 10.1002/(issn)1531-8257. [DOI] [PubMed] [Google Scholar]
- Lu W, Rajapakse JC. ICA with reference. Neurocomputing. 2006;69:2244–2257. doi: 10.1016/j.neucom.2005.06.021. [DOI] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler M, Avlund K, Mortensen EL. Socio-economic position early in life, cognitive development and cognitive change from young adulthood to middle age. European Journal of Public Health. 2013;23(6):974–980. doi: 10.1093/eurpub/cks140. [DOI] [PubMed] [Google Scholar]
- Osler M, Lund R, Kriegbaum M, Christensen U, Andersen AMN. Cohort profile: The Metropolit 1953 Danish Male Birth Cohort. International Journal of Epidemiology. 2006;35:541–545. doi: 10.1093/ije/dyi300. [DOI] [PubMed] [Google Scholar]
- Petit D, Gagnon J-F, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. Journal of Psychosomatic Research. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Petit D, Montplaisir J, Lorrain D, Gauthier S. Spectral analysis of the rapid eye movement sleep electroencephalogram in right and left temporal regions: A biological marker of Alzheimer's disease. Annals of Neurology. 1992;32:172–176. doi: 10.1002/(issn)1531-8249. [DOI] [PubMed] [Google Scholar]
- Porter VR, Buxton WG, Avidan AY. Sleep, cognition and dementia. Current Psychiatry Reports. 2015;17:97. doi: 10.1007/s11920-015-0631-8. [DOI] [PubMed] [Google Scholar]
- Pótári A, Ujma PP, Konrad BN, Genzel L, Simor P, Körmendi J, et al. Bódizs R. Age-related changes in sleep EEG are attenuated in highly intelligent individuals. NeuroImage. 2017;146:554–560. doi: 10.1016/j.neuroimage.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Rask L, Bendix L, Harbo M, Fagerlund B, Mortensen EL, Lauritzen MJ, Osler M. Cognitive change during the life course and leukocyte telomere length in late middle-aged men. Frontiers in Aging Neuroscience. 2016;8:300. doi: 10.3389/fnagi.2016.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F, Gorgoni M, Lauri G, Truglia I, Cordone S, Scarpelli S, et al. De Gennaro L. In search of sleep biomarkers of Alzheimer's disease: K-complexes do not discriminate between patients with mild cognitive impairment and healthy controls. Brain Sciences. 2017;7(5):51. doi: 10.3390/brainsci7050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspectives on Psychological Science. 2015;10(1):97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer's disease. Acta Neurologica Scandinavica. 2003;108:90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- Teasdale TW. The Danish draft board's intelligence test, Børge Priens Prøve: Psychometric properties and research applications through 50 years. Scandinavian Journal of Psychology. 2009;50(6):633–638. doi: 10.1111/j.1467-9450.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- Trotti LM. REM sleep behaviour disorder in older individuals: Epidemiology, pathophysiology and management. Drugs and Aging. 2010;27(6):457–470. doi: 10.2165/11536260-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller KL, Mortensen EL, Avlund K, Osler M, Fagerlund B, Lauritzen M, Jennum P. Subjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognition. Sleep Medicine. 2016;17:165–173. doi: 10.1016/j.sleep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Moller AA, Lauer CJ, Stolz S, Schreiber W, Dose M, Krieg JC. Nocturnal sleep in Huntington's disease. Journal of Neurology. 1991;238:203–208. doi: 10.1007/bf00314781. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. The Lancet Neurology. 2014;13(10):1017–1028. doi: 10.1016/s1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.