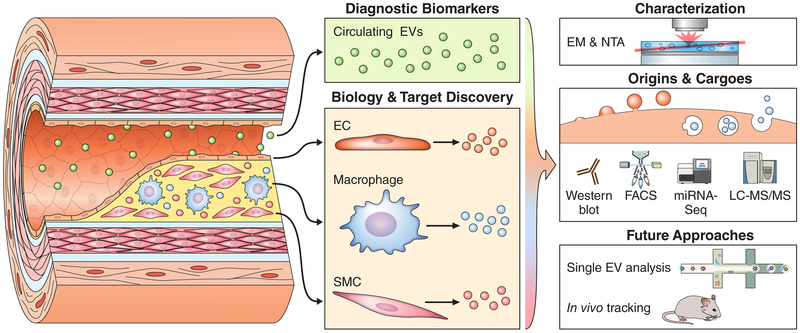
Figure 1. Blood- and Tissue-Derived Extracellular Vesicles (EVs) in Atherosclerosis.
Blood-derived circulating populations of EVs (green) are a key component of the “liquid biopsy” and may act with endocrine-like function. These EVs carry a host of detectable and stable putative diagnostic biomarkers in many cardiovascular diseases. In contrast, EVs ensnared in vascular tissues originate from all the constitutive cell types, such as endothelial cells (ECs), macrophages, or vascular smooth muscle cells (SMCs). These tissue-resident EV populations act as mediators of paracrine signaling and cell-cell cross talk, and contribute directly to calcification of atherosclerotic plaques. Their proximity to and direct action on the tissue of interest make them a rich source for novel biological insights, mechanisms of homeostasis/disease, and drug target discovery. Because EVs have context/cargo-dependent protective or harmful effects, careful characterization of the size, intracellular origins (exosomes through multi-vesicular bodies or microvesicles via budding), and RNA/protein cargoes of these EVs is essential to understanding their form and function. This is frequently achieved by the application of well-established optical techniques, including electron microscopy (EM) and nanoparticle tracking analysis (NTA), as well as molecular approaches such as Western blotting, fluorescence-activated cell sorting (FACS), transcriptomics, or liquid chromatography tandem mass spectrometry (LC-MS/MS). Upcoming technologies could allow both examination of EV composition on a vesicle-by-vesicle basis and tracking of individual EV populations within humans or animal models of disease.