Figure 1: Chemosensing by intestinal tuft cells regulates the tuft-ILC2 circuit.
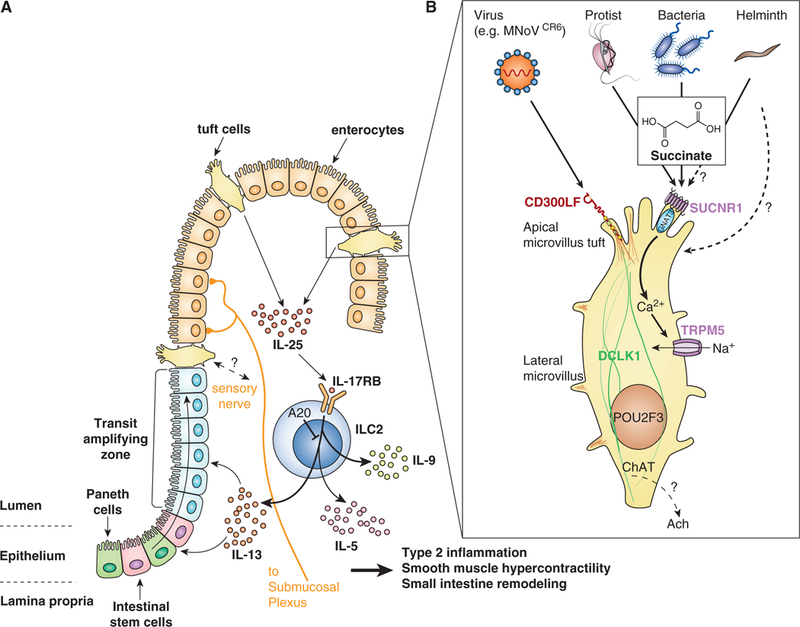
(A) Intestinal Tuft-ILC2 circuit. Tuft cells constitutively express Il25, which acts on group 2 innate lymphoid cells (ILC2s) in the lamina propria to induce production of canonical type 2 cytokines IL-5, −9, and −13, which collectively drive all aspects of innate type 2 inflammation, including eosinophilia and intestinal remodeling. IL-13 in particular signals in undifferentiated epithelial cells to bias their lineage commitment towards tuft and goblet cells, leading to hyperplasia of both cell types and driving the feed-forward tuft-ILC2 circuit. The circuit is amplified but yet unknown mechanisms when helminths or protists are sensed by tuft cells. Deletion of the signaling repressor A20 from ILC2s also amplifies the circuit and leads to chronic type 2 inflammation in the small intestine.
(B) Chemosensing. Tuft cells sense succinate secreted from Tritrichomonas protists and perhaps also bacteria and helminths. Signaling through the G protein coupled succinate receptor SUCNR1 induces an intracellular Ca2+ flux that opens the cation channel TRPM5, leading to influx of Na+ and depolarization of the cells. How cellular depolarization regulates tuft cell effector functions remains unknown, but may include release of neurotransmitter (e.g. acetylcholine (ACh)) that acts on nearby neurons. Tuft cells also express the murine nororvirus receptor CD300LF and are the host reservoir for chronic infection by the CR6 strain of norovirus. It is not clear if and how tuft cells sense this infection.
