Abstract
Background
Acne vulgaris, a chronic inflammatory disease of the pilosebaceous unit associated with socialisation and mental health problems, may affect more than 80% of teenagers. Isotretinoin is widely recognised as a very effective treatment for severe acne; however, it may cause adverse effects.
Objectives
To assess efficacy and safety of oral isotretinoin for acne vulgaris.
Search methods
We searched the following databases up to July 2017: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO and LILACS. We updated this search in March 2018, but these results have not yet been incorporated in the review. We also searched five trial registries, checked the reference lists of retrieved studies for further references to relevant trials, and handsearched dermatology conference proceedings. A separate search for adverse effects of oral isotretinoin was undertaken in MEDLINE and Embase up to September 2013.
Selection criteria
Randomised clinical trials (RCTs) of oral isotretinoin in participants with clinically diagnosed acne compared against placebo, any other systemic or topical active therapy, and itself in different formulation, doses, regimens, or course duration.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 31 RCTs, involving 3836 participants (12 to 55 years) with mild to severe acne. There were twice as many male participants as females.
Most studies were undertaken in Asia, Europe, and North America. Outcomes were generally measured between eight to 32 weeks (mean 19.7) of therapy.
Assessed comparisons included oral isotretinoin versus placebo or other treatments such as antibiotics. In addition, different doses, regimens, or formulations of oral isotretinoin were assessed, as well as oral isotretinoin with the addition of topical agents.
Pharmaceutical companies funded 12 included trials. All, except three studies, had high risk of bias in at least one domain. Attrition bias was high in 20 trials, selective reporting bias was high in 12 trials, and performance bias was high in 11 trials.
Oral isotretinoin compared with oral antibiotics plus topical agents
These studies included participants with moderate or severe acne and assessed outcomes immediately after 20 to 24 weeks of treatment (short‐term). Three studies (400 participants) showed no evidence that isotretinoin decreases trial investigator‐assessed inflammatory lesion count more than antibiotics (RR 1.01 95% CI 0.96 to 1.06), with only one serious adverse effect found, which was Stevens‐Johnson syndrome in the isotretinoin group (RR 3.00, 95% CI 0.12 to 72.98). However, we are uncertain about these results as they were based on very low‐quality evidence.
Isotretinoin may slightly improve (by 15%) acne severity, assessed by physician's global evaluation (RR 1.15, 95% CI 1.00 to 1.32; 351 participants; 2 studies), but resulted in more less serious adverse effects (67% higher risk) (RR 1.67, 95% CI 1.42 to 1.98; 351 participants; 2 studies), such as dry lips/skin, cheilitis, vomiting, nausea (both outcomes, low‐quality evidence).
Different doses/therapeutic regimens of oral isotretinoin
For our primary efficacy outcome, we found three RCTs, but heterogeneity precluded meta‐analysis. One study (154 participants) reported 79%, 80% and 84% decrease in total inflammatory lesion count after 20 weeks of 0.05, 0.1, or 0.2 mg/kg/d of oral isotretinoin for severe acne (low‐quality evidence). Another trial (150 participants, severe acne) compared 0.1, 0.5, and 1 mg/kg/d oral isotretinoin for 20 weeks and, respectively, 58%, 80% and 90% of participants achieved 95% decrease in total inflammatory lesion count. One 24‐week RCT of participants with moderate acne compared isotretinoin at (a) continuous low dose (0.25 to 0.4 mg/kg/day), (b) continuous conventional dose (0.5 to 0.7 mg/kg/day), and (c) intermittent regimen (0.5 to 0.7 mg/kg/day, for one week in a month). Continuous low dose (MD 3.72 lesions; 95% CI 2.13 to 5.31; 40 participants; one study) and conventional dose (MD 3.87 lesions; 95% CI 2.31 to 5.43; 40 participants; one study) had a greater decrease in inflammatory lesion counts compared to intermittent treatment (all outcomes, low‐quality evidence).
Fourteen RCTs (906 participants, severe and moderate acne) reported that no serious adverse events were observed when comparing different doses/therapeutic regimens of oral isotretinoin during treatment (from 12 to 32 weeks) or follow‐up after end of treatment (up to 48 weeks). Thirteen RCTs (858 participants) analysed frequency of less serious adverse effects, which included skin dryness, hair loss, and itching, but heterogeneity regarding the assessment of the outcome precluded data pooling; hence, there is uncertainty about the results (low‐ to very low‐quality evidence, where assessed).
Improvement in acne severity, assessed by physician's global evaluation, was not measured for this comparison.
None of the included RCTs reported birth defects, but oral isotretinoin is contraindicated during pregnancy due to known teratogenic effects.
Authors' conclusions
Evidence was low‐quality for most assessed outcomes.
We did not find any clear evidence from RCTs that isotretinoin improves acne severity compared with standard oral antibiotic and topical treatment when assessed by a decrease in total inflammatory lesion count, but it may slightly improve physician‐assessed acne severity. Only one serious adverse event was reported in the isotretinoin group, which means we are uncertain of the risk of serious adverse effects; however, isotretinoin may result in increased minor adverse effects.
Heterogeneity in the studies comparing different regimens, doses, or formulations of oral isotretinoin meant we were unable to undertake meta‐analysis. Daily treatment may be more effective than treatment for one week each month. None of the randomised studies in this comparison reported serious adverse effects, or measured improvement in acne severity assessed by physician's global evaluation. We are uncertain if there is a difference in number of minor adverse effects, such as skin dryness, between doses/regimens.
Evidence quality was lessened due to imprecision and attrition bias. Further studies should ensure clearly reported long‐ and short‐term standardised assessment of improvement in total inflammatory lesion counts, participant‐reported outcomes, and safety. Oral isotretinoin is a well‐established treatment for severe acne, and for acne that has not responded to oral antibiotics plus topical agents. The clinical trial evidence for oral isotretinoin conducted around 30 years ago was low quality. Further trials are needed to evaluate different dose/regimens of oral isotretinoin in acne of all severities.
Plain language summary
How effective and safe is a drug called 'isotretinoin', taken via tablet, for acne vulgaris?
Review question
How effective and safe is isotretinoin, taken in a tablet for people with acne? We reviewed the evidence about the effect of isotretinoin when compared either to itself at a different dose, to placebo (an identical but inactive treatment), or to other systemic (oral or injected medicines that work throughout the entire body) or topical (applied to the outside of the body) therapies. Eligible participants had to have been diagnosed with acne by a doctor.
Background
Acne is a persistent inflammatory disease that can affect more than 80% of teenagers. Acne (including blackheads, whiteheads, and pimples) mostly appears on the face, but can also appear on the back and chest. Mental health problems, depression, and suicidal thoughts have been associated with acne. Isotretinoin, a currently widely used therapy derived from vitamin A, transformed acne treatment. However, it may cause adverse effects and has been associated with still uncertain psychiatric events and inflammatory bowel disease.
Study characteristics
We searched the medical literature up to July 2017 and included 31 studies, involving 3836 dermatology outpatients worldwide. There were twice as many males than females; their ages ranged from 12 to 55 years old. Acne severity ranged from mild to severe, although most participants had severe acne.
The pharmaceutical industry funded 12 included studies.
We found studies that compared oral isotretinoin versus placebo or other treatments such as antibiotics. In addition, different doses, regimens (course of medical treatment), or formulations of oral isotretinoin were assessed, as well as oral isotretinoin with the addition of topical agents.
Key results
Three studies compared oral isotretinoin versus any oral antibiotic plus any topical agent given to participants with moderate or severe acne for between 20 to 24 weeks. Their outcomes were measured straight after treatment stopped.
There was no difference between therapies in decreasing the number of inflamed lesions (an area of an organ or tissue that has been damaged by disease or trauma). In one participant, isotretinoin led to the development of Stevens‐Johnson syndrome (a serious disease where skin reacts severely, often in response to medication); there were no serious side effects in the other group. However, we are uncertain of these results because they were based on very low‐quality evidence.
When assessed by a doctor, the severity of acne may be slightly improved by isotretinoin, but it may cause more side effects such as inflamed lips, dry skin, or nausea (low‐quality evidence).
Fourteen studies compared different doses/courses of oral isotretinoin between 12 to 32 weeks. Participants had mainly severe or moderate acne.
Two studies, each comparing three different doses of isotretinoin at 20 weeks, found a greater improvement (measured by inflammatory lesion counts) with the higher dose (low‐quality evidence). A third study showed that continuous (daily) low dose and continuous (daily) conventional dose may improve acne more than intermittent therapy, measured at 24 weeks (low‐quality evidence). Conventional dose isotretinoin reduced inflammatory lesion counts more than low dose, but this was based on very low‐quality evidence, indicating uncertainty.
During treatment (from 12 to 32 weeks) or follow‐up after end of treatment (up to 48 weeks), no serious side effects occurred in 14 studies analysing different doses of isotretinoin (low‐quality evidence). Doctor‐measured severity of acne was not assessed in this comparison. Less serious side effects, including skin dryness, hair loss, and itching, were assessed in 13 studies, but we are uncertain if there were any differences between groups (low‐ to very low‐quality evidence, where assessed).
No study reported birth defects.
Quality of the evidence
The overall quality of evidence for all of our key outcomes was low, due to serious limitations of study design and the limited amount of data. Thus, the identified clinical trials neither support nor challenge the established place of oral isotretinoin in acne treatment.
Summary of findings
for the main comparison.
| Oral isotretinoin compared with oral antibiotics plus topical agents for acne | ||||||
|
Patient or population: participants with moderate and severe acne Settings: outpatient Intervention: oral isotretinoin Comparison: oral antibioticsa plus topical agentsb | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral antibiotics plus topical agents | Oral isotretinoin | |||||
|
Improvement in acne severity assessed by a decrease in total inflammatory lesion count, measured in participants who were treated for a minimum period of 16 weeks (Changes from baseline in total inflammatory lesion count and number of participants who cleared inflammatory lesion) 20 to 24 weeks |
86 per 100 | 87 per 100 (83 to 91) |
1.01 (0.96 to 1.06) | 400 patients (3 RCTs) |
⊕⊝⊝⊝ very lowc | ‐ |
|
Frequency of serious adverse effects 20 to 24 weeks |
0d See comment |
0d See comment |
RR 3.00 (0.12 to 72.98) | 400 participants (3 RCTs) |
⊕⊝⊝⊝ very lowe | There was only one serious adverse effect in the intervention group (Stevens‐Johnson syndrome). There were no serious adverse effects in the control group |
|
Improvement in acne severity assessed by physician's global evaluation 20 to 24 weeks |
78 per 100 | 90 per 100 (78 to 103) |
1.15 (1.00 to 1.32) | 351 participants (2 RCTs) | ⊕⊕⊝⊝ lowf |
‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOral antibiotics were tetracycline hydrochloride, minocycline and doxycycline (one RCT for each one)
bTopical agents were adapalene (associated with oral tetracycline), azelaic acid (associated with minocycline) and adapalene/benzoyl peroxide gel (associated with doxycycline)
cQuality of the evidence was downgraded by three levels due to:
- very serious limitations of design ‐ two levels (high risk of reporting bias in one study, Gollnick 2001, and high risk of performance and attrition bias in all three studies, Gollnick 2001; Oprica 2007; Tan 2014)
- serious indirectness ‐ one level (two studies (Gollnick 2001; Tan 2014) measured our primary efficacy outcome by assessing a decrease in nodules and cysts or only in nodules, not in all inflammatory types of acne lesions (papules plus pustules, nodules and cysts))
dThe low frequency of events did not allow estimating assumed and correspondent risks.
eQuality of the evidence was downgraded by three levels, from high to very low, due to:
- very serious limitations of design ‐ lack of blinding of participants and personnel and high risk of attrition bias in all three analysed studies (Gollnick 2001; Oprica 2007; Tan 2014) and selective reporting of events in one study (Gollnick 2001)
- serious imprecision (the wide confidence interval of the effect included both beneficial and harmful clinically important differences of 25% between interventions)
fQuality of evidence was downgraded by two levels, from high to low, due to:
- very serious limitations of design ‐ lack of blinding of participants and personnel and high risk of attrition bias in both analysed studies (Gollnick 2001; Tan 2014) and selective reporting of events in one study (Gollnick 2001)
Background
Please see our glossary in Table 2 for an explanation of medical terms used throughout the text.
1. Glossary of Medical Terms.
| Medical term | Explanation |
| Apoptosis | A programmed cell death where the causes are normal and abnormal biochemical mechanisms occurring inside apoptotic cells. Apoptosis may be a result of the vital cell turnover or a consequence of cancer, neurodegenerative disease, ischaemia or autoimmune disorders |
| Angular stomatitis | An inflammation which affects the corners of the mouth |
| Arthralgia | Pain in one or more joints of the body |
| Blepharoconjunctivitis | The combination of conjunctivitis (inflammation of the conjunctiva, which is the inner surface of the eyelid) with blepharitis (inflammation of the skin in the outer surface of eyelids) |
| Case‐control | A study which consists of recognising individuals who have the outcome (e.g. a specific disease) of interest (cases) and those who do not have that same outcome (controls). The study looks back to find out if individuals (cases and controls) had an exposure of interest. The exposure could be a drug or other therapeutic intervention as well as an environmental or behavioural factor. The 2 groups are then compared to see if there is a difference in exposure |
| Cheilitis | An inflammation of the lips. Cheilitis usually presents with dry lips, cracking or peeling of the lips and flaking of the skin of the lips |
| Cutaneous | Related to the skin |
| Dermis | The layer of skin between the epidermis (outer layer of skin) and subcutaneous tissues |
| Desquamation | The spontaneous detachment of the more superficial layers of the skin |
| Discoid dermatitis | Also known as 'discoid eczema', 'microbial eczema', 'nummular eczema', 'nummular dermatitis', or 'nummular neurodermatitis', it is one of the many forms of dermatitis and presents with characteristic round or oval‐shaped itchy lesions resembling the shape of a coin |
| Epistaxis | Nasal bleeding |
| Erector pili muscle | Very small muscles attached to hair follicles contract to make the hair shaft become erect. This may cause ejection of the sebum, which is forced through the hair follicle to the surface |
| Facial dermatitis | People with this condition present with facial erythema and flaking (especially of the skin around the mouth and nose) |
| Follicular hyperkeratinisation | A disorder of the cells lining the inside of a hair follicle. These cells usually shed from the skin lining at normal intervals. The dead cells however become cohesive because of an excess of keratin (a natural protein found in the skin), and they do not shed onto the skin's surface, blocking the hair follicle |
| Hair follicle | A very small cavity in the skin that produces hair |
| Hyperkeratinisation | An alteration of the skin cell detachment process, which is reduced as a consequence of an excessive production of keratin, the protein present in the most superficial layers of skin |
| Hypertriglyceridaemia | The elevation of blood concentrations of triglycerides, which may increase risk of stroke and heart attack |
| Idiopathic intracranial hypertension (IIH) | A neurological condition characterised by increased intracranial pressure (pressure around the brain) in the absence of a tumour or other diseases. The main symptom is headache, but nausea, vomiting, pulsatile tinnitus (buzzing in the ears synchronous with the pulse), double vision, and other visual symptoms may also occur. A consequence of IIH is swelling of the optic disc in the eye, with the possibility of progression to vision loss if IIH is untreated (Binder 2004) |
| Inflammatory bowel disease (IBD) | A group of inflammatory alterations of the colon and small intestine. The main types of IBD are Crohn's disease and ulcerative colitis. Although very different diseases, both have as symptoms abdominal pain, diarrhoea, vomiting, weight loss, rectal bleeding, and severe internal cramps in the pelvic region. IBD often causes symptoms that may limit quality of life, but it is rarely fatal on its own |
| Innate and acquired immune responses | Innate immune responses are immediate and nonspecific mechanisms of response to micro‐organisms in a generic way, with the aim of protecting the host from invading micro‐organisms, such as bacteria and viruses. Acquired immune responses occur later; they are triggered by innate immunity and are specific to a micro‐organism or a molecule from a micro‐organism. They also enable a stronger response in defence as well as immunological memory |
| Microcomedones | An early acne lesion that appears with the plugging of a hair follicle by the following: skin cells lining the follicle becoming more cohesive (they are shed and accumulate in the pore instead of flowing out onto the skin); or an excess of sebum and keratin (a natural protein found in the skin) inside the follicle |
| Micronised | The property of having a very reduced average diameter, measured only by micrometer. Usually this term refers to the process involved in the production of pharmaceutical particles |
| Mucocutaneous | Related to mucosa and skin |
| Pharmacokinetic | The analysis of all process which happen in the body since the initial administration of a drug until its total excretion, including its way of action on target tissues |
| Pilosebaceous unit | A structure consisting of a hair shaft within a hair follicle to which the erector pili muscle and sebaceous glands are attached |
| Polymorphic | The characteristic of having or passing through some stages of development |
| Psychosis | A mental state characterised by a detachment from reality. People with psychosis can have hallucinations, delusional beliefs, unusual or bizarre behaviour, personality changes, and thought disorder. Several central nervous system diseases, from both external poisons and internal physiologic illness, are causes of psychosis |
| Pyogenic granuloma | A benign cell growth of the skin which is composed of numerous small blood vessels. This type of skin lesion usually is smaller than 2,5 cm, appears in a few weeks, and may easily bleed |
| Sebaceous gland | These are microscopic glands in the skin, usually found in hair‐covered areas of the body (greatest abundance on the face and scalp), which are part of the pilosebaceous unit. They secrete an oily/waxy matter (sebum), which lubricates the skin and hair. Sebum is deposited inside the hair follicles and arrives at the skin surface along the hair shaft |
| Triglycerides | One type of fat which is present in the blood |
| Xeroderma | Another term for 'dry skin'. Signs and symptoms include scaling, itching, and cracking of the skin |
| Xerosis | Dryness of the skin |
Description of the condition
Acne vulgaris is a chronic inflammatory disease of the pilosebaceous unit, which consists of a hair shaft, hair follicle, erector pili muscle, and sebaceous gland (Rocha 2014). Acne lesions predominantly affect the face and, to a lesser extent, the back and chest (Thiboutot 2009). The cause of this disease is attributed to four major factors that interact in complex ways to result in the appearance of acne lesions:
increased excretion of sebum by sebaceous glands inside the hair follicle (seborrhoea);
follicular hyperkeratinisation, which results in the formation of a plug of sebum and keratin called a microcomedone;
bacterial hypercolonisation within the pilosebaceous unit (mainly by the micro‐organism Propionibacterium acnes); and
consequent innate and acquired immune reactions triggering inflammation in affected follicles (Gollnick 2003; Kim 2005; Kurokawa 2009).
Although it is well‐established that microcomedones precede all acne lesions and the general causal mechanisms have been identified, the initial trigger for acne is not fully understood (Gollnick 2003). It is only known that inflammatory events precede hyperkeratinisation (Thiboutot 2009).
The diagnosis is clinical. Acne lesions are polymorphic and characterised by open comedones (blackheads), closed comedones (whiteheads), and inflammatory lesions (Zeichner 2016). Inflammatory lesions are more severe lesions and may take the form of papules (pinheads), pustules (pimples), or nodulocystic lesions (large nodules) (Katsambas 2014). The latter lesion type develops deeper within the dermis than the first two (Plewig 2000). Inflammatory lesions occur in acne when, due to extensive and continuous sebum production and inflammation, the follicular sac of the microcomedone ruptures into adjacent tissue (Kurokawa 2009). The surrounding dermis is affected by inflammation and becomes damaged (Gollnick 2003). Hyperpigmentation and scarring usually follows more severe acne lesions, but this may happen even after superficial lesions in those with scar‐prone skin (Holland 2004). Grading is useful in the clinical assessment of acne, and there are many grading scales, though none are universally accepted. Lesion counts are usually essential for clinical trials, but not for daily clinical practice (Layton 2010).
According to epidemiological surveys around the globe, acne is the most common reason for visiting a dermatologist (SBD 2006; Stern 2004). The global burden of disease study from 2013 found that, among skin diseases, acne was the second leading cause of disability, second only to dermatitis (Karimkhani 2017). A peak in prevalence of acne appears between 16 and 20 years (Augustin 2011; Shen 2012). In population‐based epidemiological studies which focus on adolescents, acne may affect more than 80% of the evaluated teenagers (Amado 2006; Ghodsi 2009; Tan 2007). The detrimental effects on quality of life in those with acne are now well recognised, and they are comparable to those caused by other chronic diseases, such as diabetes, asthma, and arthritis (Mallon 1999). Mental health problems, social impairment, depressive symptoms, and even suicidal thoughts have been described in association with acne, mainly in older adolescents with severe forms of the disease (Halvorsen 2011; Yazici 2004). In the teenage years, acne is more prevalent in young men; boys are also more prone to severe acne (Ghodsi 2009; Uslu 2008). However, acne prevalence is higher in girls if the analysis covers only the first years of adolescence (Aksu 2012; Kilkenny 1998). Prevalence studies from the last 15 years emphasise that acne vulgaris should no longer be considered a disease restricted to teenagers (Goulden 1999). Currently, there is good evidence that acne can be a problem beyond the teenage years in as many as 50% of individuals (Thiboutot 2009), and women are more affected than men 20 years or older (Collier 2008). Heredity not only influences susceptibility to acne, but it is also a prognostic factor (Ghodsi 2009). Family history of acne is associated with earlier occurrence, increased number of retention lesions (comedones), and treatment difficulties (Ballanger 2006). Ethnicity plays a role in the frequency and severity of acne; in studies involving ethnic groups, adolescent Caucasians have higher prevalence of acne than those of African or Asian descent (Cheng 2010).
Description of the intervention
The drug isotretinoin (13‐cis‐retinoic acid) is derived from vitamin A (Layton 2009). It is available for topical and oral administration. Oral isotretinoin was approved by the US Food and Drug Administration for nodulocystic acne in 1982 and introduced into the United Kingdom in 1983 (Leyden 2014). Since then, it has revolutionised the treatment of acne and, three decades later, remains the most clinically effective anti‐acne therapy according to physician opinion (Layton 2010). When isotretinoin was first introduced, it was almost exclusively used in those with severe nodular acne (Jones 1983). Nowadays, with the experience acquired in clinical management of oral isotretinoin, use of the drug has been widened to include those with a tendency to scarring and those who show no improvement with appropriate topical antimicrobial or retinoid‐like therapies and long‐term oral antibiotics (Cunliffe 1997; Layton 2010). Most physicians prescribe a daily dose of oral isotretinoin that varies from 0.5 to 1.0 mg/kg body weight (Del Rosso 2012); this dose results in approximately 85% of people who receive it becoming clear of acne within 16 weeks (Nast 2010). The remainder of people who receive it need about five or six months to achieve a complete response at this dose (Lehucher‐Ceyrac 1999), and fewer than 1% of them may require up to 12 months of continuous treatment to be clear of their acne (Zouboulis 2003). Treatment regimens usually begin at 0.5 mg/kg/day and may be increased to 1.0 mg/kg/day, but some centres start treatment at the higher dose, which provides optimal benefit (Layton 2010). Because pharmacokinetic evaluations showed that the absorption rate can be doubled with the concomitant presence of fat in the intestine, the advice is to take the capsules together with the main meal of the day (Colburn 1983; Webster 2013). Whether starting on a higher or lower dose, physicians usually adjust the dose over the course of the treatment, considering the response and the presence of side effects (Rademaker 2013a). The treatment duration varies from 16 to 30 weeks, with a mean of between 16 and 20 weeks (Leyden 2014). There is no cumulative dose effect, but there is a definite effect of both dose and therapy duration: post‐therapy relapse is minimised by doses that reach a total of at least 120 mg/kg (Rademaker 2013a). There is no added benefit of exceeding 150 mg/kg (Layton 2009). The duration of therapy is adjusted to produce a 90% clearance of acne lesions, which is followed by four weeks of maintenance, with the aim of consolidating the treatment before withdrawing the drug (Harms 1986).
How the intervention might work
Isotretinoin is the only therapy that targets all the primary causal factors involved in acne (Leyden 2014). Oral isotretinoin, unlike antibiotics, does not act directly on microbial cells (Layton 2009). It markedly reduces the sebum excretion rate and the sebaceous gland size (Nast 2010). By reducing sebum secretion, the drug consequently decreases the follicular hyperkeratinisation and alters the microenvironment within the duct, providing greater Propionibacterium acnes (P. acnes) suppression than that seen with topical or oral antibiotics (King 1982). The drastic reduction in the P. acnes population contributes to the reduction in acne inflammation (Coates 1997). Oral isotretinoin also modifies inflammatory activity at the cellular level (Falcon 1986) and normalises exaggerated toll‐like receptor‐mediated innate immune responses in acne (Dispenza 2012). Today, it is already known that, during a course of oral isotretinoin, the effects of the drug on acne pathogenesis correlate with the pattern of skin gene regulation (Rademaker 2013a). Just after the commencement of treatment, oral isotretinoin activates tumour suppressor genes in skin; there is induction of apoptosis and cell cycle arrest within sebaceous glands (Nelson 2009a). Within about eight weeks of treatment, there is also a downregulation of genes related to lipidic metabolism and an upregulation of genes that encode proteins from the extracellular matrix, such as collagens and fibronectin (Nelson 2009b).
Why it is important to do this review
Oral isotretinoin has many side effects. Soon after its launch on the market, the use of isotretinoin was associated with a number of psychiatric side effects: mood changes, depression, suicidal thoughts, and psychoses (Hazen 1983). Although some studies have attempted to explain these adverse effects, they remain controversial and unclear. Psychiatric events associated with isotretinoin are considered by other authors to be rare and no greater than the background incidence (Ferahbas 2004). The occurrence of idiosyncratic reactions however persists as a possibility (Magin 2005).
Mucocutaneous and cutaneous changes are the most frequent clinical adverse effects during isotretinoin therapy. They are expected, dose‐dependent, and seldom interfere with the physician's management of the condition (Rademaker 2013a). Cheilitis, xeroderma, facial dermatitis, discoid dermatitis, and blepharoconjunctivitis can usually be minimised by regular use of lip balms, eye lubricants, and moisturisers (Layton 2010). Flaring of acne lesions may occur in up to 6% of people early in the course of treatment with isotretinoin, with clinical importance in half of these (Clark 1995). Mild elevation of liver enzymes in liver function tests and fasting plasma lipids, uncommonly above the normal range, are seen in almost all those treated with isotretinoin. The discontinuation of the drug promotes a rapid return to pretreatment levels (Jones 1983;).
More uncommon side effects are headache (which may uncommonly be an early symptom of idiopathic intracranial hypertension), as well as muscle and joint pain (Hull 2000). Recently, the association of oral isotretinoin with the development of inflammatory bowel disease has been raised. Case‐control studies, however, could not consistently confirm this association (Bernstein 2009; Crockett 2010; Etminan 2013). Among its many other side effects, isotretinoin is teratogenic, which means that exposure to it during pregnancy can induce abnormalities of physiological development (Zomerdijk 2014). Approximately 20% of foetal exposures to isotretinoin may result in spontaneous abortion (Dai 1992; Lammer 1985). The risk of mental and physical birth defects associated with oral isotretinoin is 18% to 28% (Dai 1992; Lammer 1985). Any level of exposure seems to be a potential cause of malformation since there is no safe level of exposure (Sladden 2007). The most common deformities are craniofacial and cardiac (Bérard 2007; Schaefer 2010).
Due to the issue of isotretinoin teratogenicity, a Cochane review on the efficacy and safety of minocycline in acne (Garner 2012), which analysed an open randomised controlled trial comparing oral isotretinoin with a combined oral minocycline and topical azelaic acid regimen, has suggested that the minocycline and azelaic acid regimen is a safer option for women with nodular acne. In the reviewed trial, there were fewer adverse effects with the combination minocycline plus azelaic acid, and satisfactory percentage reductions in lesion counting occurred in both intervention groups during the therapy phase. Also, the onset of improvement was similar for both therapies.
Although isotretinoin is currently widely used in the treatment of acne, its efficacy and safety have not yet been assessed in a Cochrane systematic review.
The plans for this review were published as a protocol 'Oral isotretinoin for acne' (Costa 2011).
Objectives
To assess the efficacy and safety of oral isotretinoin for acne vulgaris.
Methods
Criteria for considering studies for this review
Types of studies
We evaluated all randomised controlled trials (RCTs) examining either the efficacy or safety, or both, of oral isotretinoin in people with acne vulgaris.
We did not include cluster‐randomised trials in our analysis, as we intended to analyse effects of oral isotretinoin on an individual basis. Also, we did not consider cross‐over randomised trials: oral isotretinoin produces a long‐term remission which hinders the definition of an adequate wash‐out period between interventions.
Types of participants
Our review included all those with acne vulgaris who had been clinically diagnosed by a physician.
Types of interventions
We considered oral isotretinoin at any dose, course duration, or follow‐up time, compared either to itself at a different dose, to placebo, or to other systemic or topical active therapies. We have also analysed oral isotretinoin versus oral isotretinoin plus systemic or topical active therapies.
Types of outcome measures
Primary outcomes
Improvement in acne severity assessed by a decrease in total inflammatory lesion count, measured in participants who were treated for a minimum period of 16 weeks.
Frequency of serious adverse effects.
Secondary outcomes
-
Improvement in acne severity assessed by the following tools:
Participant's self‐assessment of acne severity; and
Physician's global evaluation of acne severity.
Changes in quality of life (QoL) assessed using a validated instrument.
Frequency of less serious adverse effects.
Dropout rates.
We classified an adverse effect as serious if it was: fatal, life threatening, permanently disabling, or required hospitalisation.
The following cut‐off time points were defined for outcomes, where data were available:
short‐term follow‐up: data measured within 48 weeks after randomisation;
long‐term follow‐up: data measured 48 weeks after randomisation.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 11 July 2017 using strategies based on the draft strategy for MEDLINE in our published protocol (Costa 2011). This review fully incorporated these search results.
the Cochrane Skin Group Specialised Register, using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2017, Issue 6, in the Cochrane Library, using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946), using the strategy in Appendix 3;
Embase via Ovid (from 1974), using the strategy in Appendix 4;
PsycINFO via Ovid (from 1806), using the strategy in Appendix 5; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982), using the strategy in Appendix 6.
A further three reports of trials were identified by a search update conducted up to 14 March 2018. One was a secondary reference to a previously included study (Shetti 2013), one study was added to Studies awaiting classification and one was added to Ongoing studies. If appropriate, these latter two studies will be incorporated into the review at the next update.
Trials registries
We (CSC, RR and EB) searched the following trials registries up to 3 July 2018 using the terms 'acne' and 'isotretinoin':
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch); and
the EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
Adverse effects
We examined our included and excluded studies for common adverse effects of oral isotretinoin. In addition, we searched the following databases up to 17 September 2013 for nonrandomised studies (case‐control and cohort) on adverse effects of isotretinoin, using an amended version of the Cochrane Skin standard adverse effects search strategy and our intervention terms:
MEDLINE via Ovid (from 1946), using the strategy in Appendix 7; and
Embase via Ovid (from 1974), using the strategy in Appendix 8.
Searching other resources
We searched reference lists from retrieved studies for further references to relevant trials.
We handsearched issues of the Journal of Investigative Dermatology, Archives of Dermatology (JAMA Dermatology after January 2013), and the British Journal of Dermatology which contained conference proceedings from 1975 up to 3 July 2018.
We contacted pharmaceutical companies and experts in the field for information on relevant ongoing or unpublished studies.
Data collection and analysis
We extracted data and recorded it using data extraction forms, which were developed and piloted by two authors (CSC and EB).
We entered the data into the Cochrane RevMan 5 software and performed forest plots when available data permitted (Review Manager 2014).
Selection of studies
Two of three authors (CSC, EB, and RR) independently assessed the titles and abstracts of studies retrieved in the search in order to ascertain whether or not they represented potentially relevant trials. Based on this first assessment, we obtained the full text of all potentially relevant articles. Any disagreements were resolved by a third author.
Data extraction and management
Two of us (CSC and EB) independently extracted data using data extraction forms, with any disagreements being resolved by a third author (RR). Where it was not available, we emailed authors of studies to request data of interest. We compiled the following information from the included studies:
publication details (e.g. year, country, authors);
study design;
setting, inclusion/exclusion criteria, randomisation method, allocation concealment, blinding, and other issues relating to bias;
population data (e.g. age, severity of the acne);
interventions (details of dose, therapeutic regimen, and duration);
outcome measures (scale and time points of measurement)
dropouts;
duration of follow‐up; and
types of data analysis (e.g. imputation, modified intention‐to‐treat, intention‐to‐treat).
We then populated Characteristics of included studies tables for each included study with the extracted information.
Assessment of risk of bias in included studies
Two authors (CSC and RR) independently assessed the methodological quality of included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). A third author (RR) resolved any disagreements. For each 'Risk of bias' domain and specific question detailed below, we assigned a 'low', 'high', or an 'unclear' risk of bias. We reported on the following:
(a) random sequence generation; (b) adequate concealment of allocation; (c) blinding of participants and personnel; (d) blinding of outcome assessment; (e) incomplete outcome data; (f) selective outcome reporting; and (g) other potential threats to validity.
We considered each study as having: 1. low risk of bias, when we detected a low risk of bias for all key domains ‐ listed from (a) to (f) above; 2. an unclear risk of bias, if one or more key domains had an unclear risk of bias; and 3. high risk of bias, where we verified the presence of high risk of bias for one or more key domains within the study.
Measures of treatment effect
We summarised estimates of treatment effect with 95% confidence intervals (CI) for each comparison. We reported dichotomous outcomes as risk ratios (RRs). We reported continuous outcomes as the mean difference (MD).
Unit of analysis issues
Our unit of analysis was the individual participant. We did not include cluster‐randomised trials in our analysis. Also, we did not consider cross‐over trials; oral isotretinoin produces a long‐term remission which hinders definition of an adequate wash‐out period between interventions. We evaluated included studies with more than two groups of intervention with the following approach: we outlined multiple pairwise comparisons of the groups of the study and analysed the pairs of comparisons which were relevant to our review (Higgins 2011).
Dealing with missing data
We asked for additional information from authors when we detected missing or unavailable data. If missing data did not allow the study to be statistically analysed, we only presented and discussed the available results within the main text of the review.
Assessment of heterogeneity
We treated clinical and methodological between‐study variance as potential causes of the heterogeneity among the studies. In the presence of substantial heterogeneity, we analysed studies separately and presented them using a narrative approach.
We analysed statistical diversity by checking the estimates of treatment effect. We used the forest plots produced by Review Manager, version 5.3.5 (Review Manager 2014), and the I² statistic to identify the percentage of total variation across studies due to heterogeneity (Higgins 2003), rather than due to chance. We considered an I² statistic value higher than 50% as substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
We contacted study authors regarding reasons for the non‐reporting of data outcomes and also sought unpublished data from our included studies. We performed searches for protocols and other versions of our included trials and sought to identify duplicate publication.
Data synthesis
We used Review Manager 5 software (RevMan 2013) to summarise data. We pooled data with a random‐effects model when studies were considered to be methodologically and clinically similar. We assessed methodological or clinical heterogeneity among studies using the I² value, where greater than 50% meant significant heterogeneity (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
If possible, we intended to perform subgroup analysis to consider the following:
severity of acne;
treatment duration;
different doses and regimens;
degree of improvement in acne severity assessed by a percentage reduction in total inflammatory lesion count;
age of the participants (preadolescents vs adolescents vs adults ); and
gender.
Sensitivity analysis
Due to the low number of trials in the meta‐analyses, we could not carry out a sensitivity analysis by excluding trials of low and moderate risk of bias, as intended.
'Summary of findings' table
We created a 'Summary of findings' table for the most relevant comparison of clinical practice (oral isotretinoin versus oral antibiotics plus topical agent). The two primary outcomes (‘Improvement in acne severity assessed by a decrease in total inflammatory lesion count’ and ‘Frequency of serious adverse effects’) and one key secondary outcome ('Improvement in acne severity assessed by physician's global evaluation') were considered. For our predetermined primary and key secondary outcomes, we then used the five GRADE parameters (inconsistency, risk of bias, imprecision, indirectness, and publication bias) to assess the quality of the evidence (Guyatt 2011). We applied the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions by entering the data into GRADEpro software (GRADEpro). We described the rationale for all decisions regarding the upgrade or the downgrade of the quality of the studies in the footnotes, where we also wrote comments to assist readers' understanding of the results and assessments of our review (Higgins 2011).
Results
Description of studies
We systematically described the details of the included, excluded, awaiting classification, and ongoing studies in the following tables: Characteristics of included studies, Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Results of the search
RCTs (efficacy and safety analysis)
The electronic searches for randomised controlled trials (RCTs) returned 449 records. We added to these references another five records from handsearching and 31 records from trials registers. After removing duplicates, we evaluated titles and abstracts (if available) of 464 records. We excluded 404 references based on titles and abstracts. We obtained the full text of the remaining 60 records. We excluded a further 10 full‐text records (reporting nine studies). We identified two ongoing trials. We added one study to the awaiting classification section, as results from this trial, when identified at a late stage at the last search update, would not impact the conclusions of our review. Finally, we included 47 records reporting 31 studies in the qualitative synthesis. We entered data from seven RCTs in our quantitative synthesis (meta‐analysis). For a further description of our screening process, see the study flow diagram (Figure 1).
1.
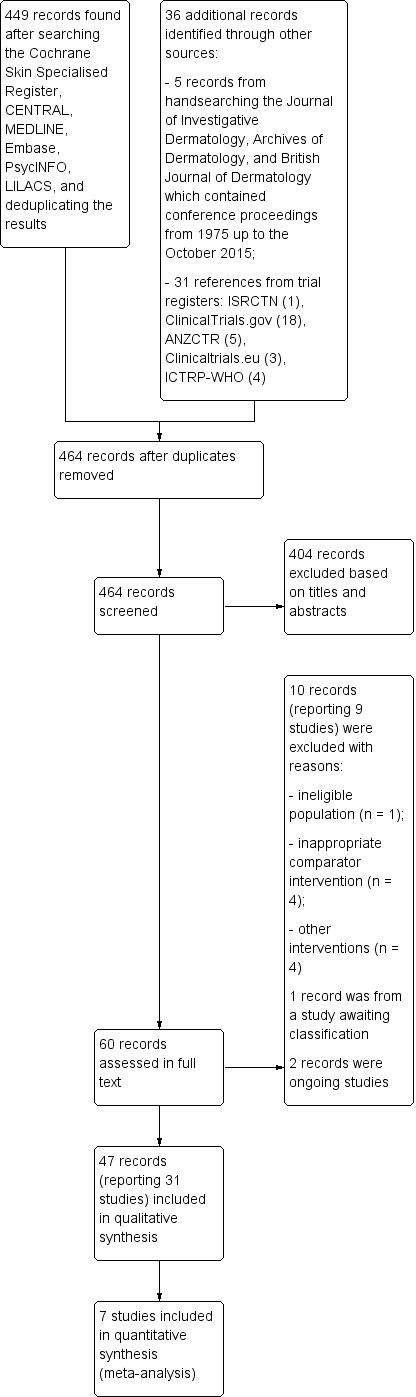
Study flow diagram.
Nonrandomised studies (safety analysis)
The additional search for nonrandomised studies (case‐control and cohort) reporting serious adverse effects of oral isotretinoin for acne returned 349 records. After removing one duplicate, we analysed 348 references on the basis of titles and abstracts. Of these, 318 were eliminated and 30 were evaluated in full text. From the 30, eight nonrandomised studies were considered for the safety analysis (Figure 2); these nonRCTs are included under Additional references. The characteristics of these included nonrandomised studies are detailed in Table 3, and the safety data from these nonRCTs are presented in the Discussion (Summary of main results).
2.
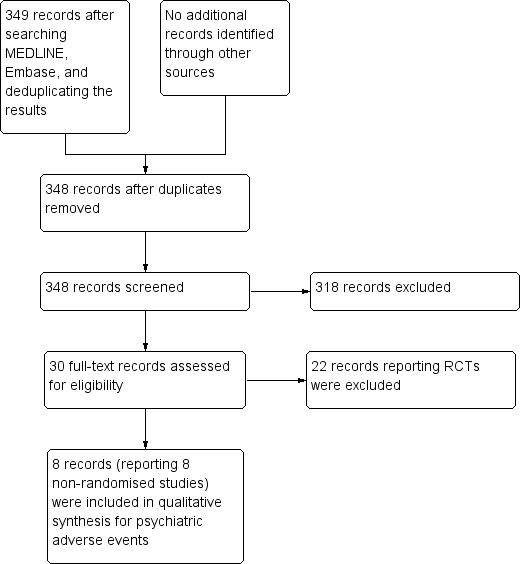
Study (nonRCT, cohort and case‐control) flow diagram.
2. Characteristics of nonrandomised studies (considered for safety analysis).
| Study | Methods | Participants | Interventions / Exposures |
Outcomes (Psychiatric adverse effects) |
Summary Findings (Frequencies of psychiatric adverse effects) |
Assessment of the risk of bias (Turner 2013) |
| Azoulay 2008 | Design: Case‐control cross‐over Duration of the study: not provided Period of recruitment: from January 1, 1984, and December 31, 2003 |
Setting: Québec, Canada Initial cohort: 30,496 participants Cases (n = 126):
Inclusion criteria: having been exposed to at least one oral isotretinoin prescription between January 1, 1984, and December 31, 2003 and diagnosed or hospitalised for depression, with an antidepressant prescription in the 30 days following the diagnosis or hospitalisation; being covered by the Régie de l' Assurance Maladie du Québec (RAMQ) drug plan for at least 12 months prior to the date of depression diagnosis (index date); having at least 1 diagnosis of acne vulgaris at any time prior to the index date * Exclusion criteria: having received an antidepressant prescription in the 12 months prior to index date |
Exposure group: oral isotretinoin use in a five‐month period immediately prior to the index date Control group: oral isotretinoin use out of the 5‐month risk period (considering a 2‐month wash‐out period) prior to the index date |
Incidence of a first diagnosis or hospitalisation for depression in each specific time window Dose response of oral isotretinoin on the incidence of depression Outcomes assessments occurred by linkage of diagnosis codes and antidepressant prescriptions with medical records |
Adjusted relative risk of depression associated with oral isotretinoin use was 2.68 (95% CI 1.10 to 6.48). There were no statistically significant differences in incidence of depression before and after Canadian label change warning about isotretinoin possible psychiatric risks There was no significant association between a cumulative dose range and the occurrence of depression |
Selection bias: High risk Although authors had reported an adjusted analysis for some covariates, the study did not consider either acne severity or time with disease as confounders Performance bias (all outcomes): High risk Comment: As the study had a retrospective design, probably personnel and participants were not blinded regarding the exposition/drug Detection bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of the outcome assessors Attrition bias (all outcomes): Unclear risk Comment: There was no information about missing data or the potential for data to be missing to permit judgment. Selective reporting bias: Low risk Comment: no protocol available; however, there was an adequate report of outcomes listed in methods section Other bias: High‐risk The study used an instrument with a poor accuracy to measure outcomes: record linkage across electronic databases. Also, the study had presented only a case‐cross‐over analysis and this design had a potential risk of bias related to the possibility of carry‐over effect in participants of nonexposed groups, despite the 2‐month wash‐out period |
| Chia 2005 | Design: Prospective cohort Duration of the study: not clearly provided Period of recruitment: between October 1998 and December 2001 Factors influencing the choice of treatment: history of previous treatment failure, participant/parent preference, out‐of‐pocket medical cost, ability/willingness to comply with treatment requirements for frequent visits and phlebotomy, participant/parental concerns about adverse drug effects, and objections to oral contraceptive therapy |
Setting: United States, two centres at Missouri: Departments of Dermatology at Saint Louis University, Saint Louis, and at University of Missouri, Columbia. Both were outpatient clinics, one urban/hospital affiliated and the other suburban/community affiliated Gender:
The study provided no information regarding duration of acne in enrolled participants Inclusion criteria: male and female participants between the ages of 12 and 19; being presented for treatment of moderate to severe inflammatory and cystic acne Exclusion criteria: history of or current DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) Axis I diagnosis; prior use of or allergy to isotretinoin, and pregnancy |
Group 1: oral isotretinoin at approximately 1 mg/kg per day, rounded to the nearest 20 mg Group 2: conservative therapy defined as a topical antibiotic, topical retinoid, and twice‐daily administration of an oral antibiotic Duration of interventions: not provided |
Frequency of CES‐D (The Center for Epidemiologic Studies Depression Scale) scores of 17 or higher, which were suggestive of clinically significant depression. The CES‐D scale is validated in adolescents and considered more sensitive and less specific than other depression instruments Incidence of suicidal ideation in all participants who scored 17 or higher in CES‐D, assessed by interviewing participants with the mood disorders portion of the Structured Clinical Interview for DSM‐IV Axis I (major mental disorders) Measurements occurred at baseline and after completing 3 to 4 months of follow‐up |
101 subjects completed the study (49 in Group 1; 52 in Group 2). After adjustment for baseline CES‐D and participant gender, there was not a significant association between treatment status (isotretinoin therapy versus conservative therapy) and follow‐up CES‐D score (prevalence at this time point) suggestive of clinically significant depression (odds ratio (OR), 1.1; 95% CI, 0.23 to 5.6). Also intention‐to‐treat analysis did not modify this result (OR, 1.3; 95% CI, 0.31 to 5.7) CES‐D scores of 17 or higher (> 16) at the 3 or 4 months of follow‐up suggesting new‐onset of depression (incidence measurement) occurred in 4.1% (2 cases) of participants in the oral isotretinoin group, and in 3.8% of participants in the conservative therapy group (RD 0.3; 95% CI, ‐7.7 to 8.3). The difference between the groups was not statistically significant, even after performing an intention‐to‐treat analysis No participant had suicidal ideation between participants with CES‐D scores > 16 in the isotretinoin group. One subject at baseline presented suicidal ideation in the control group (an incidence of 1.4%) |
Selection bias: High‐risk Comment: The study analysed the prevalence of CES‐D > 16 at follow‐up using multiple logistic regression, with baseline CES‐D score and gender as covariates in the analysis. However, the incidence of CES‐D > 16 at follow‐up was not presented using a method for control of confounding. Other potential confounders, such as duration of acne and acne severity, were not considered in the study. Also the two different settings of recruitment (one urban/hospital‐affiliated and the other suburban/community‐ affiliated outpatient clinics) could be a confounding factor, and the analysis did not consider this concern Performance bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of participants and personnel Detection bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of the outcome assessors Attrition bias (all outcomes): Low risk Comment: An intention‐to‐treat analysis, with adjustments for baseline CES‐D and gender, presented the same results of the adjusted analysis that excluded those unavailable for follow‐up. Despite the high level of loss to follow‐up in the study, there was no potential impact of missing data for all outcomes Selective reporting bias: Low risk Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section Other bias: High risk Comment: The CES‐D is a highly sensitive screening tool, which could have overestimated the degree of mood disorder compared with other psychiatric instruments and have exaggerated the adverse effects estimates of the interventions in this study |
| Cohen 2007 | Design: Prospective cohort Duration of the study: not clearly provided Period of recruitment: not provided |
Setting: community dermatology practice in Calgary, Canada Oral isotretinoin: n = 100 (41% male) Oral antibiotics: n = 41 (34.1% male) Topical acne therapy: n = 59 (18.6% male) Age – median:
Duration of acne: not provided Acne severity: percentage of participants with moderate or severe
Inclusion criteria: being 14 years old, or older, able to provide informed consent, and not currently under pharmacological treatment for depression; not anticipating an alteration in residence during the period of the study; and providing at least two options for follow‐up contact Exclusion criteria: not provided |
Intervention group (n = 100): ‐ oral isotretinoin: dosage not specified Control group (n = 100):
|
Change in scores of Zung Depression Status Inventory from baseline in both, intervention and control groups, within 25 to 35 days of the start of acne treatment Percentage of participants having a CES‐D (Center for Epidemiologic Studies Depression Scale) score greater than 15 (indicative of clinically significant depression) at baseline and follow‐up Incidence of newly detected depression (CES‐D > 15) at follow‐up in both treatment and control groups Interviews for assessment of outcomes were 'in person' at baseline, but by telephone call at follow‐up, within 25 to 35 days of the start of therapy |
There was no statistically significant difference in Zung scores between both time points of assessment (mean difference 0.00 points; 95% CI ‐0.6 to 0.6). Also there were no significant differences between groups regarding to the mean change in Zung score (one way ANOVA; df = 1, F = 1.4, P = 0.24) There was a single participant depressed at baseline (who was not depressed on follow‐up) and two on follow‐up, on the CES‐D score, both in the isotretinoin group. Despite this, incidence of newly detected depression was not statistically significant different between isotretinoin and control groups (Fisher' exact test, P = 0.497). Both new incident depression cases were female and had moderate to severe acne. None of them presented past history of depression at baseline assessment |
Selection bias: High risk Comment: The study controlled some potential confounding variables and performed a linear regression analysis to adjust the comparison between groups for acne severity, the main potential confounding factor according to authors. However, duration of acne, another important confounding factor for the analysis of the association between oral isotretinoin use for acne and depression, was not even cited within the report Performance bias (all outcomes): High risk Comment: The study had an open design Detection bias (all outcomes): High risk Comment: The study had an open design Attrition bias: (all outcomes): Unclear risk Comment: No information regarding loss to follow‐up was provided Selective reporting bias: High risk Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section Other bias: High risk Comment: The CES‐D is a highly sensitive screening tool, which could have overestimated the degree of mood disorder compared with other psychiatric instruments and have exaggerated the adverse effects estimates of the interventions in this study. Besides this, the length of the follow‐up period was inadequate, since treatment with oral isotretinoin seldom lasts more than 8 weeks. Also, instruments used to assess outcomes were different between both time points of measurement of the study: a clinical interview at baseline, and a telephone interview at follow‐up |
| Jick 2000 | Design: Retrospective population based‐cohort (record linkage) Duration of the study: not clearly provided Periods of recruitment: not provided for United Kingdom cohort; from 1983 to 1997, Saskatchewan, Canada cohort |
Settings: two different centres: province of Saskatchewan, Canada (Canadian Saskatchewan Health Database‐CSHD) and United Kingdom (United Kingdom General Practice Research Database‐UKGPRD) Saskatchewan cohort:
United Kingdom cohort:
Duration of acne: not provided Acne severity: not provided Inclusion criteria:
Exclusion criteria: not provided |
Current exposure to oral isotretinoin or to antibiotic therapies (tetracycline, erythromycin, clindamycin, minocycline or doxycycline): from the first prescription for acne treatment through 3 months after receiving the last study drug prescription Recent exposure to oral isotretinoin or to antibiotic therapies (tetracycline, erythromycin, clindamycin, minocycline or doxycycline): having received the last study drug prescription 4 to 6 months previously Nonexposed: All other times after finishing treatment with the analised drug (oral isotretinoin or antibiotic) Detailed information regarding dose and duration of exposures/ interventions were not provided |
Prevalence rates of newly diagnosed depression or psychosis (neurotic and psychotic disorders), having person‐time as denominator and the number of cases as numerator, assessed by linkage with medical records of diagnosis codes in each computerised database
|
Saskatchewan database/cohort:
GPRD database:
In both cohorts (CSHD and UKGPRD):
|
Selection bias: High risk Comment: The study had considered in the analysis some important confounding factors (i.e. age, gender, history of psychiatric disorder) and used multiple logistic regression models. However, there was no report of data related to acne severity, which may be an independent predictor of depression, apart from drug exposure Performance bias (all outcomes): High risk Comment: As the study was a retrospective cohort, probably personnel and participants were not blinded regarding the exposure/drug Detection bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of the outcome assessors Attrition bias (all outcomes): Low risk Comment: The study had used hazard ratios to measure outcomes (incidence density), which minimise bias due to missing data in both open retrospective cohorts reported Selective reporting outcome: High risk Comment: The study did not provide RR estimates for isotretinoin exposure compared with antibiotic exposure, despite having cited this outcome measurement in methods section Other bias: High risk Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company. There was a potential under‐ascertainment of psychiatric outcomes, as researchers only performed record linkage with diagnosis codes, and not psychoactive drug prescriptions or interviews. The study used a potentially insensitive instrument to measure outcomes, record linkage across electronic databases. Also, the study had presented only a cohort cross‐over analysis of data for each one of both cohorts (CSHD and UKGPRD), since the nonexposed group in each cohort included people who developed one of the psychiatric outcomes assessed 6 months after being exposed either to oral isotretinoin or to antibiotic. This design had a potential risk of bias related to the possibility of carry‐over effects in participants of nonexposed groups in both cohorts who had received oral isotretinoin previously |
| Kaymak 2009 | Design: Prospective nonrandomised controlled trial Duration of the study: not clearly provided Period of recruitment: September 2006 to May 2007 Factors influencing the choice of treatment: previous treatment failure, the severity and duration of the acne, participant preference, and participant concerns about adverse drug effects |
Setting: One outpatient dermatology clinic of a university health centre Isotretinoin (study group): n = 37 (11 males and 25 females) Topical treatment (control group): n = 41 (9 males and 20 females) Age ‐ mean ± SD (years):
Duration of acne mean ± SD (years):
Acne severity: participants with severe, moderate or mild acne in both groups. The report did not provide numbers of participants with each one grade of acne severity for each group Inclusion criteria: not provided Exclusion criteria: not provided |
Isotretinoin group: 0.5 – 0.8 mg/kg/day of oral isotretinoin with food in two divided doses for at least 20 weeks; cumulative dose of 100 mg/kg Topical treatment group: either topical antibiotics or topical retinoids. More detailed information regarding this intervention group was not provided |
Frequency of psychopathology symptoms assessed by two instruments:
Change in mean scores of BDI, HAD‐D, HAD‐A and HAD‐T during treatment compared to baseline in both treatment groups Measurements occurred at baseline, 2, and 4 months of follow‐up |
Significantly more participants had BDI scores over 13 (considered as depressive) in the topical treatment group (10/29) compared to the isotretinoin group (4/36) at 4 months time point (P = 0.03) There were also statistically significant differences between the two treatments groups, in favour of isotretinoin, regarding the number of participants with measurements of HAD‐D higher than the range of clinically significant depression (> 8) at 2 (P = 0.01) and 4 (P = 0.02) months |
Selection bias: Low risk Comment: The study performed an adjusted analysis data for the most important confounding factors while assessing psychiatric outcomes related to oral isotretinoin for acne Performance bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of participants and personnel Detection bias (all outcomes): Low risk Comment: The author who assessed participant’s psychological status was blinded Attrition bias (all outcomes): High risk Comment: The level of loss to follow‐up in the study could lead to attrition bias, especially considering that there was a high imbalance of missing data between the two groups. The trial reported a 'per‐protocol' analysis, which did not consider data from loss to follow‐up Selective reporting bias: Low risk Comment: no protocol available; however, there was an adequate report of outcomes listed in methods section Other bias:Low risk Comment: There were no other apparent sources of bias |
| McGrath 2010 | Design: Prospective nonrandomised controlled trial Duration of the study: not clearly provided Period of recruitment: between September 2006 and September 2007 |
Setting:
Gender:
Age ‐ mean ± SD (years):
Duration of acne (years): not provided Acne severity: not clearly specified Inclusion criteria:
Exclusion criteria:
|
Isotretinoin group: 0.5 mg/kg/daily for the first 2 weeks, followed by 1 mg/kg/daily until reaching the cumulative dose of 120 mg/kg (participants who did not tolerate the 1 mg/kg/day dose received the next highest dose possible) Oral antibiotic and a topical retinoid group: lymecycline 408 mg daily (or minocycline 100 mg daily in the case of intolerance or inefficacy with previous lymecycline use)plus adapalene cream |
Occurrence of depression assessed by the Centre for Epidemiological Studies Depression Scale (CES‐D) Measurements occurred at baseline, then subsequently at 3 and 6 months, for all participants in both treatment groups. Participants in the matched community control group completed the WHOQOL‐BREF, Centre for Epidemiological Studies Depression Scale (CES‐D) and visual analogue score only once |
Depression scores over time did not present significant changes in treatment groups (F1.64 = 1.06, not significant) and there was no interaction between gender and changes in depression levels (authors included gender as a covariate in the measurements of depression, since scores were higher in women than in men at baseline) | Selection bias: High risk Comment: Despite the analysis of covariance (ANCOVA), controlling for the covariate gender, and the matched healthy control group, the study did not consider other important confounders, such as duration of acne, in the comparison over time between both acne treatment groups Performance bias (all outcomes): High risk Comment: Participants and personnel were not blinded Detection bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of the outcome assessors Attrition bias (all outcomes): High risk Comment: Authors reported a high level of loss to follow‐up: only 43.7% of participants from treatment groups provided useable data on all three time points of measurements Selective reporting: High risk Comment: Due to the high level of loss to follow‐up, authors carried out data analysis only for the first two time points according to the methods section Other bias:Low risk Comment: There were no other apparent sources of bias |
| Ng 2002 | Design: Prospective nonrandomised controlled trial Duration of the study: not clearly provided Period of recruitment: between December 1998 and March 2000 |
Setting: two private dermatology clinics and a public hospital outpatient dermatology clinic in Melbourne, Australia Isotretinoin group: n = 174 (58.6% male) Antibiotic plus topical treatment group: n = 41 (41.5% male) Age – mean ± SD (years)/range (years):
Duration of acne: not provided Acne severity: participants with severe or moderate acne in both groups. The report did not provide numbers of participants with each one of the these two grades of acne severity for each treatment group Inclusion criteria: participants between 15 and 50 years having moderate to severe acne, and able to comprehend the rating instructions and comply with the study protocol Exclusion criteria: current diagnosis of depression; concomitant use of antidepressants, corticosteroids, anabolic steroids or other depression‐inducing medications; pregnancy or breastfeeding |
Isotretinoin group (n = 174): oral isotretinoin starting at 40 mg/day, increased to a dose of 1.0 mg/kg/day over 1 month according to tolerability, and continued for a total cumulative dose of 120 mg/kg (over 5–6 months) Antibiotic plus topical treatment group: a standard course of minocycline 100–200 mg/day, titrated according to weight, response and tolerance, and topical treatment consisted of adapalene 0.1% gel, tretinoin 0.05% cream or isotretinoin 0.05% gel |
Changes in mean Beck Depression Inventory (BDI) scores during treatment compared to baseline in both groups Percentage of participants having a BDI score of 10 or greater (indicative of at least a moderate level of depressive symptoms) Rate of withdrawal from the study because of worsening of mood Measurements of outcomes occurred at baseline, 1 month, 3 months and at the end of treatment course or 6 months |
Changes in mean BDI scores over the therapy course were not significantly different between both treatment groups (P = 0.62) 18 participants from the whole sample had a BDI score of 10 or greater at baseline; the incidence of moderate depressive symptoms in the isotretinoin group remained relatively unchanged during the study and at lower levels than in the antibiotic/topical group 5 participants in the isotretinoin group (n = 174) had dropped out from the study due to depressed mood, 3 males and 2 females. Only in 2 (both male) of these participants, the relationship to isotretinoin use seemed possible. No participant was withdrawn from the antibiotic plus topical group (n = 41) because of mood changes |
Selection bias: High risk Comment: Despite having controlled potential confounding variables and made a linear regression analysis to adjust the comparison between groups, not all important confounding factors were explored by the study analysis. Duration of acne and previous duration of acne treatment were not reported. Performance bias (all outcomes): High risk Comment: The study had an open design Detection bias (all outcomes): High risk Comment: The study had an open design Attrition bias: (all outcomes): High risk Comment: Despite the intention‐to‐treat analysis of data, there was an imbalance in dropout rates between the two groups Selective reporting bias: Low risk Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section Other bias: Low risk Comment: There were no other apparent sources of bias |
| Sundstrom 2010 | Design: Retrospective population‐based cohort Duration of the study: not clearly provided Period of recruitment: between 1980 and 1990 |
Setting: Sweden 5756 participants (3613 males and 2143 females) who were aged 15 to 49 years Duration of acne: not provided Acne severity: severe acne Inclusion criteria: having had at least one course of oral isotretinoin granted by the Medical Products Agency in Sweden during the period of recruitment Exclusion criteria: not provided |
Oral isotretinoin administered in a mean dose of 44.5 mg (SD 15.7) for males and 39.2 mg (SD 13.1) for females Duration of intervention: a mean period of 6.0 months (SD 4.0) for males and 6.1 (SD 3.9) for females |
Rates of attempted and completed suicides in the different time windows of the cohort compared between themselves (before, during, and after treatment with oral isotretinoin) and with those of the general population, assessed by searching for related events between 1980 and 2001 in the national patient register of in‐hospital care and in the cause of death register | Standardised incidence ratios (comparing the study cohort with the general population) for first suicide attempts and for all attempts rose, respectively, from 0.89 (95% CI, 0.54 to 1.37) and 0.99 (95% CI, 0.65 to 1.44) three years before treatment to 1.36 (95% CI, 0.65 to 2.50) and 1.57 (95% CI, 0.86 to 2.63) in the year preceding treatment. Both ratios were highest within six months after beginning treatment: 1.93 (95% CI, 1.08 to 3.18) for first attempts and 1.78 (95% CI, 1.04 to 2.85) for all attempts. Within three years after treatment, the number of suicide attempts in the cohort was almost the same as the number observed in general population, and standardised incidence ratios were 0.97 (95% CI, 0.64 to 1.40) for first attempts and 1.04 (95% CI, 0.74 to 1.43) for all attempts. However, there was a significant increase in the standardised incidence ratio of repeated events (but not of first attempts) 11 years after treatment among female participants of the cohort: 1.36 (95% CI, 1.06 to 1.70) Females who attempted suicide after completing treatment received significantly more than one course in comparison to female participants of the cohort who never attempted treatment There was a statistically significant difference in the chance of committing another suicide attempt between participants who had their first attempt before treatment and those who made a first suicide attempt during treatment or within six months after the finish of the course Treatment with oral isotretinoin reinforced more significantly the suicidal behaviour for participants who committed the first suicide attempt during treatment, or within six months after the end, in comparison with those who first attempted before using oral isotretinoin There was an increase in rates of attempted suicide per person‐years of follow‐up within the isotretinoin Swedish cohort (cohort cross‐over analysis) from time points of measurement before and after the treatment. For first attempts, the rate difference between the year before treatment and six months following the end of treatment was the highest: 0.86 cases per 1000 person‐years (95% CI, ‐0.78 to 2.50); for all attempts; this rate difference was 0.40 (95% CI, ‐1.40 to 2.26) per 1000 person‐years. The number needed to treat for an additional harmful outcome (first suicide attempt and one additional repeated attempt) was 2300 and 5000 per year, respectively 24 participants had death by suicide (17 males and 7 females) by the end of 2001. The standardised mortality ratio for males who committed suicide within one year after treatment was 1.9 (95% CI, 0.4 to 5.4), and decreased to around one within two years after treatment. For female participants, the highest standardised mortality ratio was 1.8 (95% CI, 0.7 to 3.9), which occurred within 11 years after the treatment |
Selection bias: High risk Comment: Authors considered only age, gender, and calendar year as potential confounding factors while calculating specific rates in the general population control group. The study did not take in account the fact that presence of acne and its severity in the control group (general population) could be a potential confounder Performance bias (all outcomes): High risk Comment: As the study was a retrospective cohort, probably personnel and participants were not blinded for the exposure/drug Detection bias (all outcomes): Unclear risk Comment: The study did not provide any information regarding blinding of the outcome assessors Attrition bias (all outcomes): Unclear risk Comment: There was no information about missing data or the potential for data to be missing to permit judgment. Selective reporting bias: Low risk Comment: no protocol available; however, there was an adequate report of outcomes listed in methods section Other bias: High risk Comment: The study used a potentially insensitive instrument to measure outcomes, record linkage across electronic databases. Besides this, the exclusion of the outpatients database from the estimate of rates of attempted suicide might had underestimated the primary outcome measurement in all the cohort. Also, authors reported only a single intervention group (there was no comparison within two or more interventions in the cohort), and analysis of data had an internal cross‐over pattern, a poorer design if compared with the classical cohort |
ANCOVA: analysis of covariance ARR: adjusted relative risk BDI: Beck depression inventory CES‐D: The Center for Epidemiologic Studies Depression Scale CSHD: Canadian Saskatchewan Health Database df: degrees of freedom DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition HAD‐A: hospital anxiety and depression ‐ anxiety HAD‐D: hospital anxiety and depression ‐ depression HAD‐T: hospital anxiety and depression ‐ Turkish version RAMQ: Régie de l' Assurance Maladie du Québec SD: standard deviation UKGPRD: United Kingdom (United Kingdom General Practice Research Database) WHOQOL‐BREF: World Health Organization Quality of Life‐Brief version
Included studies
We included 31 RCTs with a parallel design, involving a total of 3836 participants. We sent emails to authors from 23 included RCTs for additional data. Despite all efforts, we could not find recent email addresses for authors of eight studies (De 2011; Leheta 2011; King 1982; Jones 1983a; Jones 1983b; Lester 1985; Peck 1982; Shetti 2013).
Design
All 31 included studies were RCTs with a parallel design. Only four included RCTs (Dhir 2008; Faghihi 2014; Wahab 2008; Webster 2014) reported clearly the entire duration of the study, and the mean duration was 23 months (ranging from 17 to 40 months).
All but two (Cumurcu 2009; Leheta 2011) of the 31 trials stated the duration of treatment phase. The time point when outcomes were measured ranged from eight to 32 weeks (mean 19.7 weeks) of therapy.
The follow‐up period after the treatment phase was clearly stated in all but 11 of the 31 included studies (Ahmad 2015; Corlin 1984; De 2011; Gollnick 2001; Kapadia 2005; King 1982; Leheta 2011; Pigatto 1986; Strauss 2001; Tan 2014; Webster 2014). Mean follow‐up after therapy ended was 26.5 weeks (ranging from four to 152 weeks).
Participants
Where reported, there were 2229 men and 1081 women across the included studies.
Most of the studies provided age data for all participants as a range, and the age of participants ranged from 12 to 55 years (Agarwal 2011; Ahmad 2015; Corlin 1984; Dhaked 2016; Faghihi 2014; Farrell 1980; Goldstein 1982; Gollnick 2001; Lee 2011; Lester 1985; Oprica 2007; Pigatto 1986; Prendiville 1988; Rademaker 2013b; Strauss 2001; Van der Meeren 1983; Tan 2014; Wahab 2008; Webster 2014). Four included RCTs had reported age data only as a mean and by group without respective ranges. These mean values ranged from 19.9 to 28.5 years (Akman 2007; Cumurcu 2009; Jones 1983a; Strauss 1984). One included RCT provided age data for all participants only as mean, and the value was 25 years (standard deviation not provided) (King 1982). Another included study only reported the percentage of participants in each age range, with 81.5% of them being between 16 to 25 years (Dhir 2008). In Shetti 2013, authors only cited that participants were of either gender and more than 18 years. There was no information regarding age and gender of participants in five studies (De 2011; Jones 1983b; Kapadia 2005; Leheta 2011; Peck 1982). Six included studies did not report demographic data for all initially randomised participants. These studies described age and gender data only for participants who did not have missing data (Agarwal 2011; Akman 2007; Oprica 2007; Rademaker 2013b; Strauss 1984; Strauss 2001).
Only nine included trials reported mean duration of disease (Faghihi 2014; Goldstein 1982; Gollnick 2001; Lee 2011; Lester 1985; Oprica 2007; Strauss 1984; Van der Meeren 1983; Wahab 2008), and the mean duration of acne reported by these studies ranged from three to 12.9 years.
Fifteen trials exclusively involved participants with severe acne (Corlin 1984; De 2011; Dhir 2008; Farrell 1980; Goldstein 1982; Gollnick 2001; Lester 1985; Peck 1982; Pigatto 1986; Prendiville 1988; Strauss 2001; Strauss 1984; Tan 2014; Van der Meeren 1983; Webster 2014), and two of them (Strauss 2001; Webster 2014) included only participants with recalcitrant severe nodular acne (a total of 1527 participants). Nine studies enrolled participants with both moderate and severe acne (Akman 2007; Dhaked 2016; Faghihi 2014; Jones 1983a; Jones 1983b; Kapadia 2005; Oprica 2007; Shetti 2013; Wahab 2008). Leheta 2011 included participants with mild to moderate acne. Two trials recruited participants with all grades of acne severity: mild, moderate, and severe (Agarwal 2011; Ahmad 2015), and another enrolled only participants with moderate acne (Lee 2011). Cumurcu 2009 and King 1982 did not mention any data about acne severity. Rademaker 2013b studied low dose oral isotretinoin for participants who had persistent mild grade acne.
Sample sizes
Sample sizes of included studies varied from 16 to 925 participants.
Settings
Twelve studies were from the 1980s and 16 were from 2001 to 2017. The 31 included trials involved dermatologic outpatients from Africa (Leheta 2011), Asia (Agarwal 2011; Ahmad 2015; De 2011; Dhaked 2016; Dhir 2008; Faghihi 2014; Kapadia 2005; Lee 2011; Shetti 2013; Wahab 2008), Europe (Akman 2007; Corlin 1984; Cumurcu 2009; Gollnick 2001; Jones 1983a; Jones 1983b; King 1982; Oprica 2007; Pigatto 1986; Prendiville 1988; Van der Meeren 1983), North America (Farrell 1980; Goldstein 1982; Lester 1985; Peck 1982; Strauss 1984; Strauss 2001; Tan 2014; Webster 2014) and Oceania (Rademaker 2013b).
Ten studies were multicentric and involved more than two centres in the same country (Akman 2007; Corlin 1984; Goldstein 1982; Kapadia 2005; Leheta 2011; Strauss 1984; Strauss 2001; Tan 2014; Van der Meeren 1983; Wahab 2008) and two studies enrolled participants from multiple centres, with locations in different countries (Gollnick 2001; Webster 2014). Fifteen trials took place in a single centre (Agarwal 2011; Ahmad 2015; Cumurcu 2009; De 2011; Dhaked 2016; Dhir 2008; Faghihi 2014; Farrell 1980; Jones 1983b; Lee 2011; Oprica 2007; Peck 1982; Pigatto 1986; Rademaker 2013b; Shetti 2013), and four studies involved two centres in the same country (Jones 1983a; King 1982; Lester 1985; Prendiville 1988).
Only one of the included RCTs was not published in English: Corlin 1984 had two reports, both in German.
Interventions
RCTs tested oral isotretinoin for acne vulgaris through the following comparisons:
Oral isotretinoin versus oral antibiotics plus topical agents: oral isotretinoin versus azelaic acid cream plus minocycline (Gollnick 2001), oral isotretinoin versus tetracycline plus topical adapalene gel (Oprica 2007), and oral isotretinoin versus adapalene/benzoyl peroxide gel plus doxycycline (Tan 2014);
Oral isotretinoin versus oral isotretinoin plus topical agents: clindamycin 1% daytime plus adapalene 0.1% at bedtime (Dhir 2008), and 5% dapsone gel (Faghihi 2014);
Oral isotretinoin versus 0,1% tretinoin cream plus 5% benzoyl peroxide gel (Leheta 2011);
Oral isotretinoin versus chemical peeling with trichloroacetic acid (TCA) 25% (Leheta 2011);
Oral isotretinoin versus oral isotretinoin plus oral antibiotic: azithromycin pulse (De 2011), and erythromycin (Jones 1983b);
Oral isotretinoin versus azithromycin (Wahab 2008);
Oral isotretinoin versus erythromycin (Jones 1983b);
Oral isotretinoin versus minocycline (Pigatto 1986);
Oral isotretinoin versus tetracycline (Lester 1985);
Oral isotretinoin versus dapsone (Prendiville 1988);
Oral isotretinoin versus etretinate (Goldstein 1982);
Different doses or therapeutic regimens of oral isotretinoin (Agarwal 2011; Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; Dhaked 2016; Farrell 1980; Jones 1983a; Kapadia 2005; King 1982; Lee 2011; Shetti 2013; Strauss 1984; Van der Meeren 1983);
Standard oral isotretinoin versus other formulations of oral isotretinoin: standard isotretinoin versus micronised isotretinoin (Strauss 2001), and standard isotretinoin versus isotretinoin‐Lidose (Webster 2014);
Oral isotretinoin versus placebo (Peck 1982; Rademaker 2013b).
Outcomes
Ten included RCTs addressed the primary outcome, improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks), in a way which matched with that prespecified in our protocol, and we incorporated their results in this review. Five of these studies reported only continuous data for our primary efficacy outcome, such as mean change from baseline in total inflammatory lesion count for each intervention group and comparisons of mean total inflammatory lesion counts between intervention groups at different time points of assessment (Corlin 1984; Lee 2011; Lester 1985; Prendiville 1988; Strauss 2001). One study presented only dichotomous data for the outcome and provided number of participants in each intervention group who achieved a specific percentage decrease in total inflammatory lesion count (Oprica 2007). The remaining four studies reported both continuous and dichotomous data related to our primary efficacy outcome (Gollnick 2001; Strauss 1984; Tan 2014; Webster 2014). Five studies assessed the primary outcome of this review indirectly, they did not consider the sum of all inflammatory acne lesions (papules, pustules, nodules and cysts) (Gollnick 2001; Lester 1985; Prendiville 1988; Tan 2014; Webster 2014). The other five included RCTs assessed our primary outcome directly, by performing counts of all types of inflammatory acne lesions (Van der Meeren 1983), or indirectly, by taking into account only one or two types of inflammatory lesions (Farrell 1980; Goldstein 1982; Peck 1982; Pigatto 1986). However, in all these five studies, participants received acne therapies for less than 16 weeks.
All 31 included RCTs had assessed the frequency of serious (our primary safety outcome) or less serious (our secondary safety outcome) adverse effects, with the exception of King 1982 and Leheta 2011. King 1982 was the only included RCT which did not address any of the prespecified outcomes from this review. Leheta 2011 only analysed efficacy outcomes. Regarding the tools for assessing the occurrence of adverse effects: in 17 RCTs, there was no clear description of how clinical side effects were monitored and detected (Ahmad 2015; Akman 2007; Corlin 1984; De 2011; Dhaked 2016; Dhir 2008; Jones 1983a; Jones 1983b; Kapadia 2005; Lee 2011; Lester 1985; Peck 1982; Pigatto 1986; Prendiville 1988; Shetti 2013; Van der Meeren 1983; Wahab 2008); in three RCTs, the safety outcomes were assessed by subjective report of participants and physician clinical assessment (Cumurcu 2009; Goldstein 1982; Gollnick 2001); two RCTs evaluated the occurrence of adverse effects only by the physician clinical assessments (Agarwal 2011; Faghihi 2014); two RCTs reported that they questioned participants generally about adverse effects (Oprica 2007) or employed a prespecified questionnaire (Farrell 1980); one RCT applied a checklist to participants with the most known clinical side effects of the drug (Strauss 1984); three RCTs conducted physician clinical assessments, applied specific questionnaires and also used the subjective report of participants (Strauss 2001; Tan 2014; Webster 2014); Rademaker 2013b analysed adverse effects via a daily diary reported by participants. All 31 included RCTs, again with the exception of King 1982 and Leheta 2011, reported they had performed laboratory assessments to monitor adverse effects related to therapy with isotretinoin. Only 12 included RCTs had available data on global frequency of less serious adverse effects (Ahmad 2015; Faghihi 2014; Goldstein 1982; Gollnick 2001; Lee 2011; Lester 1985; Pigatto 1986; Rademaker 2013b; Strauss 2001; Tan 2014; Van der Meeren 1983; Webster 2014). The remaining 17 studies only provided data related to the frequencies of each detected less serious adverse event (Agarwal 2011; Akman 2007; Corlin 1984; Cumurcu 2009; Dhaked 2016; Jones 1983a; Kapadia 2005; Strauss 1984), or did not report detailed numerical data for less serious adverse effects by intervention group (De 2011; Dhir 2008; Farrell 1980; Jones 1983b; Oprica 2007; Peck 1982; Prendiville 1988; Shetti 2013; Wahab 2008).
Fifteen included trials measured the secondary efficacy outcome of this review, improvement in acne severity evaluated by physician's global evaluation of acne severity (Ahmad 2015; Akman 2007; Dhaked 2016; Dhir 2008; Faghihi 2014; Gollnick 2001; Jones 1983b; Kapadia 2005; Lee 2011Leheta 2011; Shetti 2013; Strauss 2001; Tan 2014; Wahab 2008; Webster 2014). In seven of them (Dhaked 2016; Dhir 2008; Gollnick 2001; Kapadia 2005; Strauss 2001; Wahab 2008; Webster 2014), authors performed a subjective global evaluation of the improvement in acne and reported the number of participants from each intervention group who achieved a satisfactory result (complete, excellent or good clearing of acne lesions). Tan 2014 provided the number of participants in each group who had an improvement of at least 2 grades from baseline in the IGA (Investigator Global Assessment), a six‐point scale from 0 (clear) to 5 (very severe acne). Ahmad 2015 used the Global Acne Scoring (GAS) system, in which there was the following grading of acne severity: mild (scores 1 to 18), moderate (scores 19 to 30), severe (scores 31 to 38), and very severe (scores > 39). Ahmad 2015 provided data for this secondary outcome as the mean decrease in GAS scores with each intervention. Lee 2011 measured the outcome, physician's global evaluation of acne severity, using the Global Acne Grading System (GAGS) score (Doshi 1997). In Akman 2007, investigators assessed the outcome as the mean decrease in FDA Global Grade as described in Cunliffe 2003. Jones 1983b cited the assessment of acne grade but did not provide any other detail. Leheta 2011 and Shetti 2013 also only reported the measurement of acne severity score and Global Acne Grading System (GAGS), respectively, without further specifications.
The secondary efficacy outcome, improvement in acne severity evaluated by participant's self‐assessment, was an assessed outcome in only four included RCTs (De 2011; Prendiville 1988; Strauss 2001; Rademaker 2013b). De 2011 evaluated acne severity by participant's assessments using a 10‐point visual analogue scale (VAS), for which the authors did not provide details in the report. Prendiville 1988 cited in the methods section of the report that the authors addressed the subjective improvement in acne assessed by each participant by a visual analogue scale (VAS), ranging from –5 to +5. However, there was no report of data related to this outcome among the results section of the study. In Strauss 2001, an efficacy outcome was the participant's subjective global assessment of the improvement in acne. The study reported the number of participants from each intervention group who achieved a satisfactory result (complete, excellent, or good clearing of acne lesions) according to their self‐evaluations. In Rademaker 2013b, participants assessed the severity of their own condition by using a linear visual 10 cm scale graded from 0 to 10, with 0 being 'none' and 10 being 'very bad acne'.
Only two included RCTs (Oprica 2007; Rademaker 2013b) assessed changes in quality of life using a validated instrument. Both studies had provided to participants the Dermatology Life Quality Index (DLQI), a self‐administered questionnaire designed to quantify the impact of skin diseases on participants' quality of life (Finlay 1994). Maximum score for the DLQI questionnaire is 30, and the higher value coincides with more impairment in quality of life.
All 31 included RCTs, with exception of King 1982, Leheta 2011, and Shetti 2013 addressed dropout rates. Ten from these 28 RCTs also analysed reasons for dropouts (Corlin 1984; Cumurcu 2009; Gollnick 2001; Lee 2011; Lester 1985; Oprica 2007; Prendiville 1988; Rademaker 2013b; Tan 2014; Van der Meeren 1983).
Excluded studies
From the search for RCTs, we excluded 10 records (reporting nine studies), and the reasons for exclusion are presented in Characteristics of excluded studies tables.
Studies awaiting classification
One study is awaiting classification (Faghihi 2017). This study was screened after the final update search and will be included in any further updates of this review, as we realised it would not impact the conclusions at this late stage.
Ongoing studies
After searching for ongoing trials, we found two records from two trials (IRCT201104094310N6; IRCT2013110315246N1). We described these studies in a 'Characteristics of ongoing studies' table.
Risk of bias in included studies
Please see Figure 3, Figure 4 and Characteristics of included studies.
3.
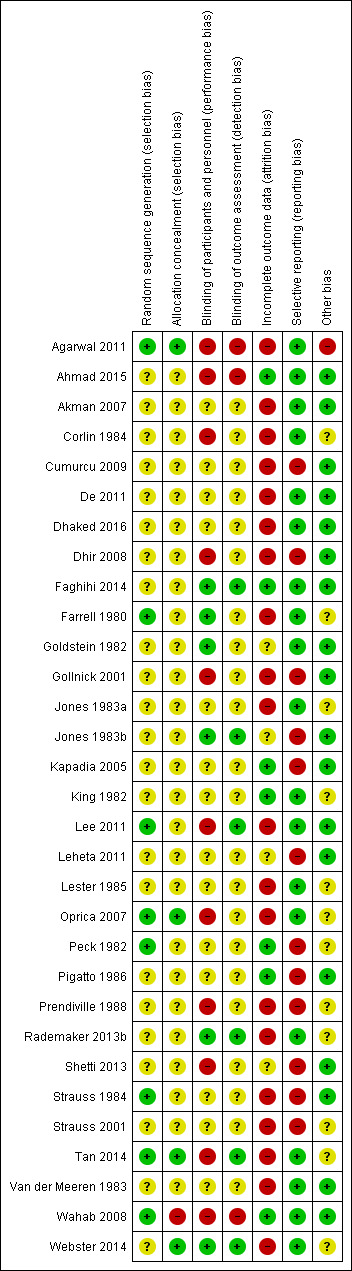
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
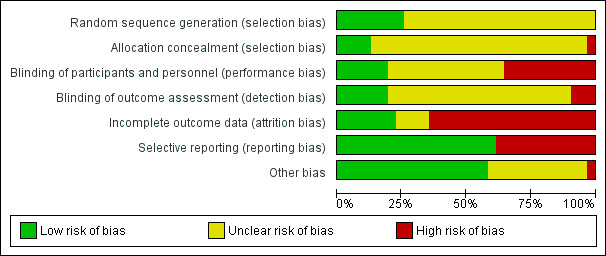
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
All 31 included trials reported being randomised; however, only eight studies described an adequate method to generate the random sequence: Agarwal 2011 adopted shuffled cards; Farrell 1980, Oprica 2007, Peck 1982; Strauss 1984 and Tan 2014 used a computer‐generated randomised code; Wahab 2008 declared by personal communication to us that they used drawing of lots; and Lee 2011 reported a restricted randomisation in a 1:1:1 ratio, also using a computer‐generated schedule. We judged these eight trials to be at low risk of bias for this domain. We considered the remaining 23 studies as presenting an unclear risk of bias for random sequence generation. From these 23 trials, two studies failed to report the method adopted to generate the random sequence (Strauss 2001; Webster 2014), despite claiming to have used methods to randomise with constraints, such as stratification and blocking. The other 21 studies did not provide any information about random sequence generation (Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; De 2011; Dhaked 2016; Dhir 2008; Faghihi 2014; Goldstein 1982; Gollnick 2001; Jones 1983a; Jones 1983b; Kapadia 2005; King 1982; Lester 1985; Lester 1985; Pigatto 1986; Prendiville 1988; Rademaker 2013b; Shetti 2013; Van der Meeren 1983). No trials were considered as at high risk of selection bias from random sequence generation.
Allocation sequence concealment
From 31 included trials, we judged 26 studies as at unclear risk of bias for the domain, since there was no statement regarding allocation concealment within their reports (Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; De 2011; Dhaked 2016; Dhir 2008; Faghihi 2014; Farrell 1980; Goldstein 1982; Gollnick 2001; Jones 1983a; Jones 1983b; Kapadia 2005; King 1982; Lee 2011; Leheta 2011; Lester 1985; Peck 1982; Pigatto 1986; Prendiville 1988; Rademaker 2013b; Shetti 2013; Strauss 1984; Strauss 2001; Van der Meeren 1983). Authors from Wahab 2008 declared, after contact by email, that they did not use any concealment method while allocating interventions, and we considered this study at high risk of bias for allocation concealment. We assessed only four trials as being at low risk of allocation sequence concealment (Agarwal 2011; Oprica 2007; Tan 2014; Webster 2014). These studies reported a 'third‐party' assignment.
Blinding
Participants and personnel (performance bias)
Fourteen included trials did not provide adequate information to permit judgement and we considered them as being at unclear risk of this type of bias (Akman 2007; Cumurcu 2009; De 2011; Dhaked 2016; Jones 1983a; Kapadia 2005; King 1982; Leheta 2011; Lester 1985; Peck 1982; Pigatto 1986; Strauss 1984; Strauss 2001; Van der Meeren 1983). From these studies, six declared a 'double‐blind' design, but did not specify who exactly was blinded among participants and personnel during the trial (King 1982; Jones 1983a; Lester 1985; Peck 1982; Strauss 1984; Strauss 2001).
Nine trials reported an open design (Agarwal 2011; Corlin 1984; Dhir 2008; Gollnick 2001; Lee 2011; Oprica 2007; Prendiville 1988; Shetti 2013; Wahab 2008), one trial (Tan 2014) declared that the study was investigator‐blinded only, and one trial compared single versus divided daily dose of isotretinoin without using a dummy capsule to ensure the blinding (Ahmad 2015). We considered these eleven studies as being at high risk of performance bias.
Only six trials had a low risk of performance bias. These trials cited the 'double‐blind' design and described accurately who was blinded among participants and personnel (Faghihi 2014; Farrell 1980; Goldstein 1982; Rademaker 2013b; Webster 2014) or described an appropriate effort to ensure blinding (Jones 1983b). None of the 31 included RCTs reported having performed any assessment of the success of blinding of participants and personnel.
Outcome assessor (detection bias)
Six of the 31 included trials described blinding of outcome assessment in an adequate way (Faghihi 2014; Lee 2011; Rademaker 2013b; Tan 2014; Webster 2014), or described an appropriate effort to ensure blinding (Jones 1983b): we considered them as at low risk of detection bias, despite the fact they had not declared any assessment of the success of blinding. We classified three trials as having high risk of bias: two trials which described clearly an open design with no blinding of the outcome assessor (Agarwal 2011; Wahab 2008), and one study which compared single versus divided daily dose of isotretinoin without the use of a placebo (Ahmad 2015). From the remaining 22 studies, five trials cited an open design and did not provide any information related to outcome assessments (Corlin 1984; Dhir 2008; Gollnick 2001; Shetti 2013; Oprica 2007), and 17 studies did not report in a precise way either who did the outcome assessments or if the people responsible for measurement or collection of all outcome data were blinded (Akman 2007; Cumurcu 2009; De 2011; Dhaked 2016; Farrell 1980; Goldstein 1982; Jones 1983a; Kapadia 2005; King 1982; Leheta 2011; Lester 1985; Peck 1982; Pigatto 1986; Prendiville 1988; Strauss 1984; Strauss 2001; Van der Meeren 1983). We judged all these 22 clinical trials as presenting an unclear risk of bias.
Incomplete outcome data
Four included studies presented an unclear risk of attrition bias: Goldstein 1982 and Jones 1983b did not report detailed numbers and reasons for missing data in each intervention group; Leheta 2011 and Shetti 2013 did not provide any information regarding loss to follow‐up to permit judgement.
We judged seven studies as being free of attrition bias: in Ahmad 2015, Faghihi 2014, and Kapadia 2005 there were no losses; King 1982, Peck 1982, Pigatto 1986, and Wahab 2008 made no mention of missing data of interest to our analysis within their reports (from Peck 1982, we considered in our review only the first phase of the study).
All of the remaining 20 included trials had a high risk of bias due to incomplete outcome data in our assessment. From these studies, seven trials performed a 'per protocol' analysis for at least the main efficacy outcome (Agarwal 2011; Akman 2007; Corlin 1984; Dhaked 2016; Dhir 2008; Farrell 1980; Oprica 2007; Strauss 2001) and performed an analysis restricted to participants who complied adequately with the protocol. Seven trials did analyses including missing data and reported reasons for attrition, and there was imbalance in numbers of dropouts and reasons for them across intervention groups (Gollnick 2001; Lee 2011; Lester 1985; Prendiville 1988; Tan 2014; Van der Meeren 1983; Webster 2014). Cumurcu 2009 reported a low and equal number of participants with missing data in both interventions groups, but there was an imbalance in reasons for it across groups. As a result, we decided to consider this trial as having a high risk of bias for attrition. Four studies reported a high loss to follow‐up, which could lead to considerable attrition bias (De 2011; Jones 1983a; Rademaker 2013b; Strauss 1984). Three of these studies had not clearly described the number of participants with missing data in each group (Jones 1983a; Rademaker 2013b; Strauss 1984).
Selective reporting
Four of the 31 included trials had an available written or published protocol (Faghihi 2014; Rademaker 2013b; Tan 2014; Webster 2014). We judged 19 trials as presenting a low of risk bias, as they presented an adequate report of results related to each one of all outcomes listed in the methods sections of published final texts or protocols (Agarwal 2011; Ahmad 2015; Akman 2007; Corlin 1984; De 2011; Dhaked 2016; Faghihi 2014; Farrell 1980; Goldstein 1982; Jones 1983a; King 1982; Lee 2011; Lester 1985; Oprica 2007; Rademaker 2013b; Tan 2014; Van der Meeren 1983; Wahab 2008; Webster 2014).
Twelve included studies had high risk of selective reporting:
There were outcomes reported with inadequate detail in six studies (Cumurcu 2009; Jones 1983b; Leheta 2011; Kapadia 2005; Prendiville 1988; Shetti 2013).
Selective choice of data for an outcome might have occurred in four trials, (Dhir 2008; Pigatto 1986; Strauss 1984; Strauss 2001), and selective reporting of subsets of the data in another one (Peck 1982).
One study might have selectively omitted one of the outcomes listed in its methods section from the report (Gollnick 2001).
Other potential sources of bias
Eighteen studies appeared to be free from other potential source of bias (Ahmad 2015; Akman 2007; Cumurcu 2009; De 2011; Dhaked 2016; Dhir 2008; Faghihi 2014; Goldstein 1982; Gollnick 2001; Jones 1983b; Kapadia 2005; Lee 2011; Leheta 2011; Pigatto 1986; Shetti 2013; Strauss 1984; Van der Meeren 1983; Wahab 2008). In one study (Agarwal 2011), there was an inappropriate administration of two other co‐interventions (pulsed oral azithromycin plus topical 1% clindamycin phosphate cream twice daily) in a similar way for all groups. We considered this as a potential risk of bias as the association with other active anti‐acne drugs could overestimate the actual effect of oral isotretinoin in intervention groups using lower doses. In 12 studies, the funding body was a pharmaceutical industry; in nine of them, the company that has first developed and marketed oral isotretinoin provided funding. Due to the possibility of inappropriate influence of funding bodies, we considered all these 12 trials as being at unclear risk of bias for the domain (Corlin 1984; Farrell 1980; Jones 1983a; King 1982; Lester 1985; Oprica 2007; Peck 1982; Prendiville 1988; Rademaker 2013b; Strauss 2001; Tan 2014; Webster 2014).
Effects of interventions
See: Table 1
1. Oral isotretinoin versus oral antibiotics plus topical agents
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
Three RCTs assessed this outcome (Gollnick 2001; Oprica 2007; Tan 2014). Overall, oral isotretinoin did not have a greater effect on improving acne severity than any combination of oral antibiotic plus topical agent after 20 to 24 weeks therapy (RR 1.01 95% CI 0.96 to 1.06; participants = 400; studies = 3; I² = 46%) (Analysis 1.1) (Figure 5). Participants in these three trials had moderate or severe acne. We decide to downgrade the quality of this body of evidence from high to very low due to very serious design limitations ‐ selective reporting in one study (Gollnick 2001) and high risk of performance and attrition bias in all three studies ‐ and serious indirectness, as two studies (Gollnick 2001; Tan 2014) measured our primary efficacy outcome by assessing a clearance of nodules and cysts or only of nodules, not of all inflammatory types of acne lesions (papules plus pustules, nodules and cysts). All these data are in Table 1.
1.1. Analysis.
Comparison 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, Outcome 1 Improvement in acne severity assessed by a decrease in total inflammatory lesion count, measured in participants who were treated for a minimum period of 16 weeks.
5.
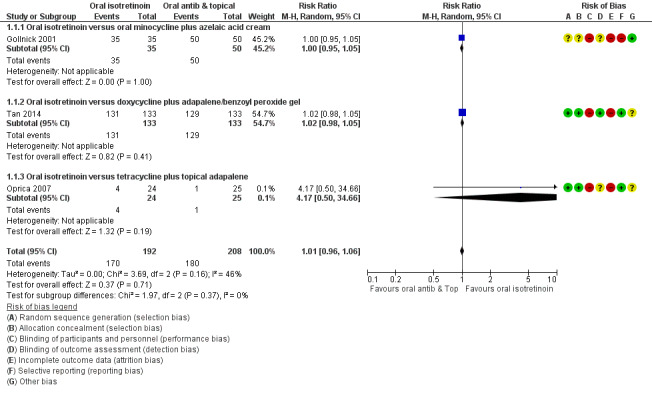
Forest plot of comparison: 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, outcome: 1.1 Improvement in acne severity assessed by a decrease in total inflammatory lesion count, measured in participants who were treated for a minimum period of 16 weeks.
As there was statistical heterogeneity in pooling effects from these three studies (I² = 46%), that had analysed different combinations of oral antibiotics plus topical agents against oral isotretinoin during different periods of treatment, we also analysed the primary efficacy outcome of this review by study. The control intervention was equivalent to oral isotretinoin in one study (RR 1.00, 95% CI 0.95 to 1.05; participants = 85) (Analysis 1.1) (Gollnick 2001), which compared oral isotretinoin with azelaic acid cream plus minocycline (AA/mino) for severe acne during 24 weeks. The quality of the evidence was downgraded to low due to the very serious design limitations in Gollnick 2001 (high risk of selective reporting, performance and attrition bias). Oral isotretinoin produced only a 2% greater decrease in total inflammatory lesion count at the end of therapy phase (Tan 2014) where the control was doxycycline plus adapalene/benzoyl peroxide gel and participants with severe acne received treatment for 20 weeks (RR 1.02, 95% CI 0.98 to 1.05; participants = 266) (Analysis 1.1). The quality of this evidence was low due to the very serious limitations of design (high risk of performance and attrition bias) in the trial conducted by Tan 2014. In Oprica 2007, which evaluated oral isotretinoin versus tetracycline plus topical adapalene for moderate and severe acne during 24 weeks, the decrease in total inflammatory lesion count was more than four times higher with oral isotretinoin but the result had a very wide confidence interval including 1 showing uncertainty (RR 4.17, 95% CI 0.50 to 34.66; participants = 49) (Analysis 1.1). This study had the quality downgraded from high to very low due to the serious imprecision (the confidence interval of the effect was very wide) and the serious limitations of design in Oprica 2007 (high risk of performance and attrition bias).
Only Oprica 2007 provided data for the outcome at a two‐month follow‐up period after the end of therapy. Participants in the oral isotretinoin group were 11.4 times more likely to be clear of acne inflammatory lesions than those who received tetracycline plus adapalene; however, the confidence interval was very wide and included 1 showing uncertainty (RR 11.44, 95% CI 0.67 to 196.30; participants = 49; studies = 1). We downgraded the quality of this evidence from high to very low due to the very serious limitation of design in the study (high risk of performance and attrition bias) and serious imprecision of the effect, which had a wide confidence interval. During the two‐month follow‐up period, participants from the tetracycline plus adapalene group applied adapalene once a day, while participants from the oral isotretinoin group did not use any maintenance therapy.
Gollnick 2001 and Tan 2014 also provided continuous data for our primary efficacy outcome. Gollnick 2001 reported a 97.1% reduction in the median number of facial papules and pustules for the oral isotretinoin group (n = 35) versus 88.2% for the azelaic acid cream plus minocycline group (n = 50) after six months of treatment (P < 0.05), which favoured the isotretinoin group. In Tan 2014, isotretinoin produced a higher decrease in nodule counts than doxycycline plus adapalene/benzoyl peroxide gel after 20 weeks of therapy (95.6 % versus 88.7 %, P < 0.01). In both studies, neither standard deviations, exact P value or a confidence interval were provided for these measurements.
Primary outcome: Frequency of serious adverse effects
Three RCTs assessed this outcome in participants with moderate or severe acne at 24 weeks follow‐up and we pooled the results in meta‐analysis (Gollnick 2001; Oprica 2007; Tan 2014). Only one serious adverse event was reported in the isotretinoin group (Stevens‐Johnson syndrome just after the beginning of treatment, which required hospitalisation for 24 days). No adverse event was reported in the control group. The risk of serious adverse effects was three times higher with oral isotretinoin than with oral antibiotics plus topical agents but the confidence interval was very wide and included 1 (RR 3.00, 95% CI 0.12 to 72.98; participants = 400; studies = 3; I² = 0%) (Analysis 1.2) (Figure 6) (Table 1). We downgraded the quality of evidence from high to very low due to very serious limitations of design (lack of blinding of participants and personnel and high risk of attrition bias in all three analysed studies (Gollnick 2001; Oprica 2007; Tan 2014) and selective reporting of events in one study (Gollnick 2001)) and serious imprecision (the wide confidence interval of the effect, including 1).
1.2. Analysis.
Comparison 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, Outcome 2 Frequency of serious adverse effects.
6.
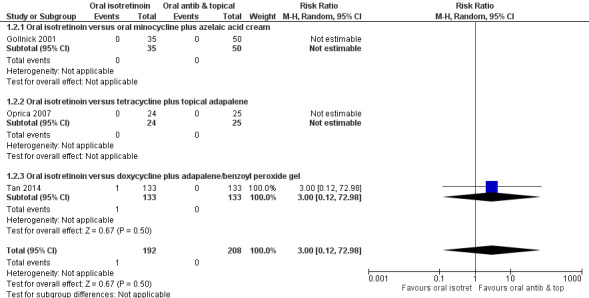
Forest plot of comparison: 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, outcome: 1.2 Frequency of serious adverse effects.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
One RCT planned to assess this outcome, but did not report the respective result (Gollnick 2001).
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Two RCTs (Gollnick 2001; Tan 2014) assessed the outcome in participants with severe acne and data from them had significant heterogeneity when pooled in meta‐analysis (I² = 68%). These two studies used quite different criteria for judging acne severity improvement, with assessment of the physician's global evaluation by different scales, which may explain the heterogeneity.
Overall, when oral isotretinoin was compared with any combination of oral antibiotic plus topical agent, the meta‐analysis showed a 15% higher improvement in acne severity with oral isotretinoin for this outcome (RR 1.15, 95% CI 1.00 to 1.32; participants = 351; studies = 2; I² = 68%) (Analysis 1.3; Table 1). The quality of the evidence was downgraded from high to very low due to very serious limitations of design (high risk of performance and attrition bias in both studies (Gollnick 2001; Tan 2014), besides selective reporting of events in Gollnick 2001) and serious imprecision related to the wide confidence interval of the effect. However, due to the significant clinical and statistical heterogeneity between these studies, we also analysed and presented the results for each one separately. In one study, oral isotretinoin had greater efficacy and produced an improvement in acne severity 22% higher than oral antibiotics plus topical agents. The quality of the evidence was low due to the very serious limitations of design cited above (RR 1.22, 95% CI 1.09 to 1.38; participants = 266) (Tan 2014). In the other study, both interventions had the same efficacy regarding this outcome (RR 1.08, 95% CI 0.97 to 1.20; participants = 85) (Gollnick 2001). The quality of evidence was low due to the very serious limitations of design in Gollnick 2001.
1.3. Analysis.
Comparison 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, Outcome 3 Improvement in acne severity assessed by physician's global evaluation.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
Only one study, Oprica 2007, assessed this outcome in participants with moderate and severe acne using the Dermatology Life Quality Index (DLQI) (Finlay 1994), a self‐administered questionnaire designed to quantify the impact of skin diseases on participants' quality of life, where higher scores meant worse quality of life. Comparing final score values between groups, the mean final value of DLQI in participants on oral isotretinoin was 0.5 lower than of those in the control group but the confidence interval was wide and included zero (MD ‐0.50, 95% CI ‐3.58 to 2.58; participants = 49; studies = 1). The quality of evidence was considered very low according to GRADE due to very serious limitations of design in this study (high risk of attrition and performance bias) and serious imprecision of the effect (very wide confidence interval, which includes the clinically important difference in the effect size of 0.5 in either direction).
Secondary outcome: Frequency of less serious adverse effects
Two RCTs assessed the outcome in participants with severe acne, and we pooled data from them in a meta‐analysis (Gollnick 2001; Tan 2014). The adverse effects considered included the following: local events (severe or persistent skin symptoms, or both, such as dry lips, dry skin, and cheilitis) and systemic events (vomiting and nausea). There was, respectively, a 90% and 66% higher frequency of less serious adverse effects in the isotretinoin group measured after four weeks (RR 1.90, 95% CI 1.03 to 3.51; participants = 85; studies = 1) (Analysis 1.4) (Gollnick 2001) and 20 weeks (RR 1.66, 95% CI 1.39 to 1.97); participants = 266 ; studies = 1) (Analysis 1.4) (Tan 2014). Oral isotretinoin therapy also had a 67% higher risk of less serious adverse effects in comparison to the use of oral antibiotics plus topical agents in the meta‐analysis of data from these studies (RR 1.67, 95% CI 1.42 to 1.98; participants = 351; studies = 2; I² = 0%) (Analysis 1.4). The quality of this body of evidence was downgraded from high to low due to very serious limitations of design in both studies (high risk of performance and attrition bias in both studies, besides high risk of reporting bias in Gollnick 2001). Additionally, we presented the data on each less serious adverse event reported among both RCTs in Table 4.
1.4. Analysis.
Comparison 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, Outcome 4 Frequency of less serious adverse effects.
3. Less serious adverse effects from RCTs. Oral isotretinoin versus itself (different formulations or combined with topical) or other active therapies.
| Adverse effects (AE) outcomes (clinical and laboratory) | Study | Oral isotretinoin versus itself (different formulations or combined with topical) or other active therapies | Estimate of effect: risk ratio, 95% CI | |
|
Intervention Number of participants who experienced the AE/Number of analysed participants |
Control Number of participants who experienced the AE/Number of analysed participants |
|||
| 1.Overall incidence ‐ measured at 1st month | Gollnick 2001 | Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 16/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months* 12/50 |
1.90 (1.03 to 3.51)a |
| 2. Overall incidence ‐ during the 20‐week therapy | Tan 2014 | Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 116/133 |
Oral doxycycline 200 mg plus adapalene 0.1% /benzoyl peroxide 2.5% gel (both once daily) 70/133 |
1.66 (1.39 to 1.97)a |
| 3. Overall incidence ‐ during the 8‐week therapy | Faghihi 2014 |
Oral isotretinoin, 20 mg once a day plus vehicle neutral gel applied on the face twice daily, both for 8 weeks 3/29 |
Oral isotretinoin, 20 mg once a day plus 5% dapsone gel applied on the face twice daily, both for 8 weeks 18/29 |
0.17 (0.06 to 0.51)a |
| 4. Overall incidence ‐ during 20‐week therapy | Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 385/460 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 403/464 |
0.96 (0.91 to 1.02) |
| 5. Overall incidence ‐ during 20‐week therapy | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 293/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 296/300 |
1.01 (0.99 to 1.03 |
| 6. Overall incidence ‐ during 16‐week therapy | Rademaker 2013b | Oral isotretinoin 5 mg once daily 28/29 |
Placebo 26/30 |
4.31 (0.45 to 41.09) |
| 7. Severe/persistent skin symptoms | Gollnick 2001 | Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 7/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 3/50 |
3.33 (0.93 to 12.01) |
| 8. Systemic side effects | Gollnick 2001 | Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 6/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 6/50 |
1.43 (0.50 to 4.07) |
| 9. Melasma‐like pigmentation | Dhir 2008 | Oral isotretinoin 20 mg twice a day for 24 weeks 5/25 |
Oral isotretinoin 20 mg twice a day along with topical clindamycin (1%) during the daytime and adapalene (0.1%) at bedtime for 24 weeks 3/25 |
1.66 (0.44 to 6.24) |
| 10. Flare‐up (during the first eight weeks of treatment) | Dhir 2008 |
Oral isotretinoin 20 mg twice a day for 24 weeks 3/25 |
Oral isotretinoin 20 mg twice a day along with topical clindamycin (1%) during the daytime and adapalene (0.1%) at bedtime for 24 weeks 6/25 |
0.50 (0.14 to 1.78) |
| 11. Dry lips | Tan 2014 | Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 66/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 9/133 |
7.33 (3.81 to 14.10)a |
| Rademaker 2013b | Oral isotretinoin 5 mg once daily 18/29 |
Placebo 3/30 |
14.73 (3.60 to 60.26) | |
| 12. Cheilitis | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 28/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 28/28 |
1.00 (0.93 to 1.07) |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 274/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 271/300 |
1.01 (0.96 to 1.06) | |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 12/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
25.00 (1.65 to 379.57)a | |
| Tan 2014 | Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 32/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 2/133 |
16.00 (3.91 to 65.42)a | |
| 13. Cheilitis or dry lips | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 15/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 13/15 |
4.43 (1.75, 11.23)a |
| 14. Xerosis (dry skin) | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 28/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 21/28 |
1.33 (1.06 to 1.65)a |
| Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 15/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
6.20 (1.98 to 19.43)a | |
| Strauss 2001 |
Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 152/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 159/300 |
0.96 (0.82 to 1.12) | |
| Rademaker 2013b | Oral isotretinoin 5 mg once daily 6/29 |
Placebo 4/30 |
1.70 (0.42 to 6.77) | |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 12/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
25.00 (1.65 to 379.57)a | |
| Tan 2014 | Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 43/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 22/133 |
1.95 (1.24 to 3.08)a | |
| 15. Pruritus | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 28/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 16/28 |
1.73 (1.25 to 2.38)a |
| Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) | |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 1/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
3.00 (0.13 to 67.06) | |
| 16. Rhinitis sicca | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 21/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 16/28 |
1.31 (0.89 to 1.93) |
| 17. Nasal dryness (dry nose) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 96/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 91/300 |
1.05 (0.83 to 1.34) |
| Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 10/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
10.00 (1.46 to 68.69)a | |
| 18. Epistaxis | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 14/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 9/28 |
1.56 (0.81 to 2.99) |
| Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 10/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
21.00 (1.34, 328.86)a | |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 122/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 103/300 |
1.18 (0.96 to 1.46) | |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 8/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
17.00 (1.09 to 265.02)a | |
| 19. Nasopharyngitis | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 23/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 17/300 |
1.35 (0.74 to 2.48) |
| 20. Sore mouth | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 12/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 5/28 |
2.40 (0.97 to 5.92) |
| 21. Dry mouth | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 3/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
3.00 (0.35 to 25.68) |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 4/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
9.00 (0.54 to 150.81) | |
| 22. Facial dermatitis | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 18/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 8/28 |
2.25 (1.18 to 4.30)a |
| 23. Dermatitis | Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 10/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 11/28 |
0.91 (0.46 to 1.79) |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 46/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 25/300 |
1.84 (1.16 to 2.91)a | |
| 24. Skin fragility | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 11/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 11/28 |
1.00 (0.5 to 1.92) |
| 25. Localised exfoliation | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 126/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 113/300 |
1.12 (0.92 to 1.36) |
| 26. Erythema | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 3/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
7.00 (0.39 to 124.83) |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 4/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
9.00 (0.54 to 150.81) | |
| 27. Erythematous eruption | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 108/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 86/300 |
1.26 (0.99 to 1.59) |
| 28. Desquamation | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 7/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
15.00 (0.93 to 241.20) |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 7/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
15.00 (0.95 to 236.42) | |
| 29. Fingertip peeling | Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 10/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 18/28 |
0.56 (0.31 to 0.98) |
| 30. Tender finger tips | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 31. Desquamation of palms and soles | Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 5/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 16/28 |
0.31 (0.13 to 0.74)a |
| 32. Eczema | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13, 68.26) |
| 33. Fissuring | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 34. Crusting of lesions | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 35. Morbiliform eruption | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
0.33 (0.01 to 7.58) |
| 36. Herpes simplex | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
0.33 (0.01 to 7.58) |
| 37. Flushing | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
1.00 (0.07 to 14.55) |
| 38. Pyogenic granuloma‐like lesions | Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 1/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
3.00 (0.13 to 67.06) |
| 39. Light intolerance | Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 1/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
3.00 (0.13 to 67.06) |
| 40. Phototoxicity | Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 0/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 1/12 |
0.33 (0.01 to 7.45) |
| 41. Alopecia | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 3/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
7.00 (0.39 to 124.83) |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 3/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
7.00 (0.40 to 122.44) | |
| Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 9/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 15/28 |
0.60 (0.32 to 1.14) | |
| 42. Dry hair | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
0.50 (0.05 to 4.94) |
| 43. Dry eyes or xerophthalmia | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 2/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
5.00 (0.26 to 96.13) |
| Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 2/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
5.00 (0.27 to 94.34) | |
| Rademaker 2013b | Oral isotretinoin 5 mg once daily 5/29 |
Placebo 2/30 |
2.92 (0.52 to 16.42) | |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 90/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 80/300 |
1.13 (0.87 to 1.45) | |
| 44. Eye irritation | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 16/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 6/28 |
2.67 (1.22 to 5.81)a |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 84/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 71/300 |
1.18 (0.90 to 1.55) | |
| 45. Eye pain | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 6/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 1/28 |
6.00 (0.77 to 46.66) |
| 46. Mild conjunctival injection (assessed only at 8 weeks) | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 7/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 7/28 |
1.00 (0.40 to 2.48) |
| 47. Conjunctivitis | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 48. Photophobia | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 49. Pterygium, right eye | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 50. Small posterior subcapsular cataract opacities | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 2/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
5.00 (0.26 to 96.13) |
| 51. Headache | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 8/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 4/28 |
2.00 (0.68 to 5.89) |
| Rademaker 2013b |
Oral isotretinoin 5 mg once daily 7/29 |
Placebo 14/30 |
0.36 (0.12 to 1.11) | |
| Strauss 2001 |
Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 40/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 48/300 |
0.83 (0.57 to 1.23) | |
| 52. Lethargy | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 13/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 4/28 |
3.25 (1.21 to 8.75)a |
| 53. Fatigue | Rademaker 2013b | Oral isotretinoin 5 mg once daily 3/29 |
Placebo 3/30 |
1.04 (0.19 to 5.62) |
| 54. Tiredness | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
0.20 (0.01 to 3.85) |
| 55. Joint pain | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 6/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 3/28 |
2.00 (0.55 to 7.22) |
| 56. Back pain | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 16/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 11/300 |
1.45 (0.69 to 3.08) |
| 57. Musculoskeletal symptoms | Rademaker 2013b | Oral isotretinoin 5 mg once daily 6/29 |
Placebo 2/30 |
3.65 (0.67 to 19.85) |
| 58. Increased thirst | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 9/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 7/28 |
1.29 (0.56 to 2.97) |
| Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
0.20 (0.01 to 3.85) | |
| 59. Increased appetite | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 6/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 1/28 |
6.00 (0.77 to 46.66 ) |
| 60. Anorexia | Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 2/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 3/12 |
0.67 (0.13 to 3.30) |
| 61. Abdominal pain | Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 3/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 4/28 |
0.75 (0.18 to 3.05) |
| 62. Nausea | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 1/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 1/28 |
1.00 (0.07 to 15.21) |
| Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 0/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 7/12 |
0.07 (0.00 to 1.05) | |
| Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 3/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 9/133 |
0.33 (0.09 to 1.20) | |
| 63. Vomiting | Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 0/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 3/12 |
0.14 (0.01 to 2.50) |
| Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 1/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 10/133 |
0.10 (0.01 to 0.77)a | |
| 64. Diarrhea | Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 0/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 2/12 |
0.14 (0.01 to 2.50) |
| 65. Infections | Rademaker 2013b |
Oral isotretinoin 5 mg once daily 11/29 |
Placebo 15/30 |
0.61 (0.22 to 1.72) |
| 66. Indigestion | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
0.33 (0.01 to 7.58) |
| 67. Decreased sweating | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 1/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
3.00 (0.13 to 68.26) |
| 68. Insomnia | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 8/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 8/28 |
1.00 (0.44 to 2.29) |
| 69. Mild psychiatric disorders | Strauss 2001 |
Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 1/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 11/300 |
0.09 (0.01 to 0.70)a |
| 70. Depressive episode (moving from BDI‐IIb scores ≤ 13 at baseline to > 13 during treatment) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 7/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 6/300 |
1.17 (0.40 to 3.43) |
| 71. Bone loss | Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 0/231 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 1/245 |
0.35 (0.01 to 8.63) |
| 72. Bone age change | Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 4/192 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 5/204 |
0.85 (0.23 to 3.12) |
| 73. Elevated serum triglycerides (> 140 mg/dL) | Goldstein 1982 |
Oral isotretinoin 1 mg/kg/day for 8 weeks 2/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 3/28 |
0.67 (0.12 to 3.69) |
| 74. Raised triglyceride levels | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 4/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
9.00 (0.53 to 153.79) |
| 75. Elevated serum triglycerides (during first 10 weeks of therapy) | Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 4/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
9.00 (0.54 to 150.81) |
| 76. Marked abnormality in serum triglycerides level (> 250 mg/dL and increase from baseline ≥ 100%) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 75/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 48/300 |
1.56 (1.13, 2.16)a |
| 77. Marked abnormality in serum cholesterol level (> 320 mg/dL and increase from baseline ≥ 50%) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 1/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 0/300 |
3.00 (0.12 to 73.35) |
| 78. Persistent elevated serum alkaline phosphatase (during therapy and follow‐up phase) | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 3/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 3/15 |
1.00 (0.24 to 4.18) |
| 79. Raised alkaline phosphatase | Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 4/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
9.00 (0.54 to 150.81) |
| 80. Marked abnormality in alkaline phosphatase level (> 190 U/L and increase from baseline ≥ 50%) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 0/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 0/300 |
Not estimable |
| 81. Persistent raised SGOTc (AST)d and SGPTe (ALT)f levels (during therapy) | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 1/15 |
0.33 (0.01 to 7.58) |
| 82. Raised ASTd or ALTf | Pigatto 1986 |
Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 0/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 5/12 |
0.09 (0.01 to 1.48) |
| 83. Marked abnormality in ALTf (SGPT)e level (> 60 U/L and increase from baseline ≥ 50%) |
Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 8/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 7/300 |
1.14 (0.42 to 3.11) |
| 84. Marked abnormality in ASTd (SGOT)c level (> 50 U/L and increase from baseline ≥ 50%) | Strauss 2001 |
Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 5/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 9/300 |
0.56 (0.19 to 1.64) |
| 85. Persistent elevated serum protein (during therapy and follow‐up phase) | Lester 1985 | Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 3/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 0/15 |
7.00 (0.39 to 124.83) |
| 86. Raised serum protein | Pigatto 1986 | Oral isotretinoin 1 mg/kg/day for 10 weeks followed by 0.5 mg/kg/day for 10 weeks 1/12 |
Minocycline 100 mg/day for 10 weeks and then reduced to 50 mg/day for 10 weeks 0/12 |
3.00 (0.13 to 67.06) |
| 87. Marked abnormality in GGTg level (> 120 U/L and increase from baseline ≥ 50%) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 7/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 4/300 |
1.75 (0.52 to 5.92) |
| 88. Marked abnormality in LDHh level (> 500 U/L and increase from baseline ≥ 50%) | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 1/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 0/300 |
3.00 (0.12 to 73.35) |
| 89. Transient abnormalities in semen evaluations | Goldstein 1982 | Oral isotretinoin 1 mg/kg/day for 8 weeks 0/28 |
Oral etretinate 1 mg/kg/day for 8 weeks 0/28 |
Not estimable |
| Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 3/15 |
0.14 (0.01 to 2.55) | |
aResults that showed a statistically significant difference between interventions
bBDI‐II (Beck Depression Inventory‐II)
cSGOT (serum glutamic oxaloacetic transaminase)
dAST (aspartate aminotransferase)
eSGPT (serum glutamic pyruvic transaminase)
fALT (alanine aminotransferase)
gGGT (gamma‐glutamyl transpeptidase)
hLDH (lactate dehydrogenase)
*The intervention groups which had lower less serious adverse event rates in each comparison are in bold.
Secondary outcome: Dropout rates
Three RCTs assessed dropout rates in participants with moderate and severe acne. We pooled data from these three trials in a meta‐analysis (Gollnick 2001; Oprica 2007; Tan 2014). Regarding dropout rates due to any reason, when oral isotretinoin was compared with any combination of oral antibiotic plus topical agent, the meta‐analysis showed a 31% lower risk of dropout in the clinical trials among participants on isotretinoin but the confidence interval included 1 showing uncertainty (RR 0.69, 95% CI 0.44 to 1.09; participants = 403; studies = 3; I² = 0%) (Analysis 1.5). The quality of this body of evidence was downgraded from high to very low (three levels) after verifying the very serious limitations of design in all three studies (high risk of performance and attrition bias in the three studies, Gollnick 2001; Oprica 2007; Tan 2014, with also high risk of reporting bias in Gollnick 2001) and serious imprecision of the effect, which had a wide confidence interval. When compared to any oral antibiotics plus topical agents, oral isotretinoin was associated with a 27%, 70% and 10% reduction in risk of dropout, respectively, due to: adverse effects (RR 0.83, 95% CI 0.30 to 2.31; participants = 403; studies = 3; I² = 0%), no improvement or lack of efficacy (RR 0.30, 95% CI 0.03 to 2.64; participants = 137; studies = 2; I² = 0%); and loss to follow‐up or no specified reason (RR 0.90, 95% CI 0.36 to 2.26; participants = 403; studies = 3; I² = 0%); however, the confidence intervals for these analyses were wide and included 1 showing uncertainty (Analysis 1.5). For all of these last three analyses, the quality of evidence was downgraded to very low due to very serious limitations of design (high risk of bias related to, at least, performance and attrition domains) in all three studies which had data on these outcomes (Gollnick 2001; Oprica 2007; Tan 2014) and serious imprecision (effects with wide confidence intervals, which included the clinically relevant difference of 25%). Additionally, we also presented the data concerning dropout rates from our 31 included RCTs in Table 5 and Table 6.
1.5. Analysis.
Comparison 1 Oral isotretinoin versus any oral antibiotic plus any topical agent, Outcome 5 Dropout rates.
4. Dropout rates from RCTs. Oral isotretinoin alone or in combination versus other active topical or systemic active therapy.
| Dropout rates | Study | Comparison | Estimate of effect: risk ratio, 95% CI | |
|
Intervention Number of participants who dropped out/Number of analysed participants |
Control Number of participants who dropped out/Number of analysed participants |
|||
| 1. Overall dropout rates | Gollnick 2001 |
Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months* 2/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 6/50 |
0.48 (0.10 to 2.22) |
| Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 7/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 6/26 |
1.17 (0.45 to 3.00) | |
| Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 59/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 51/302 |
1.16 (0.83 to 1.63) | |
| Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 60/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 70/464 |
0.86 (0.63 to 1.19) | |
| Dhir 2008 | Oral isotretinoin 20 mg twice a day for 24 weeks 5/30 |
Oral isotretinoin 20 mg twice a day along with topical clindamycin (1%) during the daytime and adapalene (0.1%) at bedtime for 24 weeks 5/30 |
1.00 (0.26 to 3.89) | |
| Faghihi 2014 | Oral isotretinoin, 20 mg once a day plus vehicle neutral gel applied on the face twice daily, both for 8 weeks 0/29 |
Oral isotretinoin, 20 mg once a day plus 5% dapsone gel applied on the face twice daily, both for 8 weeks 0/29 |
Not estimable | |
| Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
0.20 (0.01 to 3.85) | |
| Prendiville 1988 | Oral isotretinoin 40 mg daily for 16 weeks 3/20 |
Dapsone 100 mg daily for 16 weeks 3/20 |
1.00 (0.23 to 4.37) | |
| Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 17/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 28/133 |
0.61 (0.35 to 1.06) | |
| 2. Dropout rates due to improvement at 6 months | Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 1/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 0/26 |
3.00 (0.13 to 70.42) |
| 3. Dropout rates due to lack of efficacy | Gollnick 2001 |
Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 0/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 1/50 |
0.47 (0.02 to 11.27) |
| 4. Dropout rates due to treatment failure | Lester 1985 |
Oral isotretinoin 1.0 to 2.0 mg/kg/day for 16 weeks 0/15 |
Oral tetracycline 0.5 to 1 g/day for 16 weeks 2/15 |
0.20 (0.01 to 3.85) |
| 5. Dropout rates, no improvement or worsening at 4 months | Oprica 2007 |
Oral isotretinoin 1 mg/kg/day for 24 weeks 0/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 2/26 |
0.20 (0.01 to 3.97) |
| 6. Dropout rates due to adverse effects | Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 4/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 6/133 |
0.67 (0.19 to 2.31) |
| 7. Dropout rates due to adverse events | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 16/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 16/302 |
1.01 (0.51 to 1.98) |
| Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 15/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 19/464 |
0.79 (0.41 to 1.54) | |
| 8. Dropout rates due to adverse effects (local skin irritation) | Gollnick 2001 |
Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 0/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 1/50 |
0.47 (0.02 to 11.27) |
| 9. Dropout rates due to adverse effects (gastric pain) | Gollnick 2001 |
Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 0/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 1/50 |
0.47 (0.02 to 11.27) |
| 10. Dropout rates due to adverse effect (hypersensitivity reaction) | Prendiville 1988 |
Oral isotretinoin 40 mg daily for 16 weeks 0/20 |
Dapsone 100 mg daily for 16 weeks 1/20 |
0.33 (0.01 to 7.72) |
| 11. Dropout rates due to adverse effects (flare‐up) at 2 months | Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 1/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 0/26 |
3.00 (0.13 to 70.42) |
| 12. Dropout rates due to adverse effect (severe xerosis) at 4 months | Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 2/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 0/26 |
5.00 (0.25 to 99.34) |
| 13. Dropout rates due to adverse effect (gastrointestinal disturbances) at 4 months | Oprica 2007 |
Oral isotretinoin 1 mg/kg/day for 24 weeks 0/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 1/26 |
0.33 (0.01 to 7.82) |
| 14. Dropout rates due to loss to follow‐up | Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 4/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 6/133 |
0.67 (0.19 to 2.31) |
| Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 60/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 70/464 |
0.81 (0.42 to 1.53) | |
| 15. Dropout rates due to poor compliance | Gollnick 2001 |
Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 1/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 3/50 |
0.48 (0.05 to 4.39) |
| Webster 2014 | Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 8/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 5/464 |
1.61 (0.53 to 4.89) | |
| 16. Dropout rates due to non‐attendance or poor compliance | Prendiville 1988 | Oral isotretinoin 40 mg daily for 16 weeks 3/20 |
Dapsone 100 mg daily for 16 weeks 1/20 |
3.00 (0.34 to 26.45) |
| 17. Dropout rates due to loss to follow‐up, refusal of treatment or violation of the protocol | Strauss 2001 | Standard oral isotretinoin 0.85 to 1.18 mg/kg/day for 20 weeks 43/300 |
Micronised oral isotretinoin 0.32 to 0.4 mg/kg/day for 20 weeks 35/302 |
1.24 (0.82 to 1.88) |
| 18. Dropout rates due to dissatisfaction with treatment | Prendiville 1988 |
Oral isotretinoin 40 mg daily for 16 weeks 0/20 |
Dapsone 100 mg daily for 16 weeks 1/20 |
0.33 (0.01 to 7.72) |
| 19. Dropout rates due to participant's request, withdrawal of consent | Tan 2014 |
Oral isotretinoin (0.5 mg/kg/day for the first 4 weeks with escalation to 1 mg/kg/day for subsequent 16 weeks) plus vehicle gel 3/133 |
Oral doxycycline 200 mg plus adapalene 0.1%/benzoyl peroxide 2.5% gel (both once daily) 8/133 |
0.38 (0.10 to 1.38) |
| Webster 2014 | Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 15/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 15/464 |
1.01 (0.50 to 2.03) | |
| 20. Dropout rates due to other reasons (not specified) | Gollnick 2001 | Oral isotretinoin 0.5 to 0.8 mg/kg/day for 6 months 1/35 |
Oral minocycline (50 mg twice daily) plus topical azelaic acid cream (twice daily) for 6 months 0/50 |
4.25 (0.18 to 101.39) |
| Webster 2014 |
Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 4/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 10/464 |
0.40 (0.13 to 1.27 | |
| 21. Dropout rates due to other reasons (not specified) at 2 months | Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 1/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 1/26 |
1.00 (0.07 to 15.15) |
| 22. Dropout rates due to other reasons (not specified) at 4 months | Oprica 2007 | Oral isotretinoin 1 mg/kg/day for 24 weeks 1/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 0/26 |
3.00 (0.13 to 70.42) |
| 23. Dropout rates due to other reasons (not specified) at 6 months | Oprica 2007 |
Oral isotretinoin 1 mg/kg/day for 24 weeks 1/26 |
Tetracycline 500 mg twice daily plus topical adapalene 0.1% once a day for 24 weeks 2/26 |
0.50 (0.05 to 5.18) |
| 24. Dropout rates due to investigator's decision | Webster 2014 | Standard oral isotretinoin, 0.5 mg/kg/day first 8 weeks, followed by 1 mg/kg/day until week 20 2/461 |
Isotretinoin‐Lidose, 1 mg/kg/day for 20 weeks 1/464 |
2.01 (0.18 to 22.12) |
* The intervention groups which had lower dropout rates in each comparison are in bold.
5. Dropout rates from RCTs. Oral isotretinoin at different doses/schemes.
| Dropout rates | Study | Comparison | Estimate of effect: risk ratio, 95% CI | |
|
Intervention Number of participants who dropped out/Number of analysed participants |
Control Number of participants who dropped out/Number of analysed participants |
|||
| 1. Overall dropout rates | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks* 2/30 |
1 mg/kg/day, for 16 weeks 3/30 |
0.67 (0.12 to 3.71) |
|
20 mg (alternate day), for 16 weeks 1/30 |
1 mg/kg/day (alternate day), for 16 weeks 2/30 |
0.50 (0.05 to 5.22) | ||
|
20 mg (alternate day), for 16 weeks 1/30 |
1 mg/kg/day, for 16 weeks 3/30 |
0.33 (0.04 to 3.03) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 2/30 |
1 mg/kg/day, for 16 weeks 3/30 |
0.67 (0.12 to 3.71) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 2/30 |
1 mg/kg/day, (alternate day), for 16 weeks 2/30 |
1.00 (0.15 to 6.64) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 2/30 |
20 mg (alternate day), for 16 weeks 1/30 |
2.00 (0.19 to 20.90) | ||
| Akman 2007 |
0.5 mg/kg/day first 10 days, for 6 months (intermittent) 0/22 |
0.5 mg/kg/day for 1 month, afterwards 0.5 mg/kg/daily first 10 days, for 5 months (continuous first month, then intermittent) 3/22 |
0.14 (0.01 to 2.61) | |
|
0.5 mg/kg/day first 10 days, for 6 months (intermittent) 0/22 |
0.5 mg/kg/day, for 6 months 3/22 |
0.14 (0.01 to 2.61) | ||
| 0.5 mg/kg/day for 1 month, afterwards 0.5 mg/kg/day first 10 days, for 5 months (continuous first month, then intermittent) 3/22 |
0.5 mg/kg/day, for 6 months 3/22 |
1.00 (0.23 to 4.42) | ||
| Corlin 1984 | 0.05 mg/kg/day for 20 weeks 23/64 |
0.1 mg/kg/day for 20 weeks 8/62 |
2.79 (1.35 to 5.75)a | |
| 0.05 mg/kg/day for 20 weeks 23/64 |
0.2 mg/kg/day for 20 weeks 6/65 |
3.89 (1.70 to 8.92)a | ||
| 0.1 mg/kg/day for 20 weeks 8/62 |
0.2 mg/kg/day for 20 weeks 6/65 |
1.40 (0.51 to 3.80) | ||
| Cumurcu 2009 | < 0.5 mg/kg/day for 90 days 1/25 |
> 0.5 mg/kg/day for 90 days 1/26 |
1.04 (0.07 to 15.74) | |
| Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 3/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 4/20 |
0.75 (0.19 to 2.93) | |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 4/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 3/20 |
1.33 (0.34 to 5.21) | ||
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 4/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 4/20 |
1.00 (0.29 to 3.45) | ||
|
Strauss 1984 (primary reference) |
0.1 mg/kg/day for 20 weeks 4/50 |
0.5 mg/kg/day for 20 weeks 4/50 |
1.00 (0.26 to 3.78) | |
| 0.1 mg/kg/day for 20 weeks 4/50 |
1.0 mg/kg/day for 20 weeks 1/50 |
4.00 (0.46 to 34.54) | ||
| 0.5 mg/kg/day for 20 weeks 4/50 |
1.0 mg/kg/day for 20 weeks 1/50 |
4.00 (0.46 to 34.54) | ||
|
Strauss 1984 (secondary reference) |
0.1 mg/kg/day for 20 weeks 3/50 |
0.5 mg/kg/day for 20 weeks 5/50 |
0.60 (0.15 to 2.38) | |
|
0.1 mg/kg/day for 20 weeks 3/50 |
1.0 mg/kg/day for 20 weeks 5/50 |
0.60 (0.15 to 2.38) | ||
| 0.5 mg/kg/day for 20 weeks 5/50 |
1.0 mg/kg/day for 20 weeks 5/50 |
1.00 (0.31 to 3.24) | ||
| Van der Meeren 1983 | 0.5 mg/kg/day for 12 weeks 4/31 |
1.0 mg/kg/day for 12 weeks 3/27 |
1.16 (0.28 to 4.73) | |
| 2. Dropout rates due to full acne remission | Corlin 1984 |
0.05 mg/kg/day for 20 weeks 0/64 |
0.1 mg/kg/day for 20 weeks 1/62 |
0.32 (0.01 to 7.78) |
| 0.05 mg/kg/day for 20 weeks 0/64 |
0.2 mg/kg/day for 20 weeks 0/65 |
Not estimable | ||
| 0.1 mg/kg/day for 20 weeks 1/62 |
0.2 mg/kg/day for 20 weeks 0/65 |
3.14 (0.13 to 75.72) | ||
| 3. Dropout rates due to treatment failure after 12 weeks | Corlin 1984 | 0.05 mg/kg/day for 20 weeks 6/64 |
0.1 mg/kg/day for 20 weeks 2/62 |
2.91 (0.61 to 13.85) |
| 0.05 mg/kg/day for 20 weeks 6/64 |
0.2 mg/kg/day for 20 weeks 1/65 |
6.09 (0.75 to 49.20) | ||
| 0.1 mg/kg/day for 20 weeks 2/62 |
0.2 mg/kg/day for 20 weeks 1/65 |
2.10 (0.20 to 22.54) | ||
| 4. Dropout rates due to treatment failure after 12 weeks despite an increase in dose | Corlin 1984 | 0.05 mg/kg/day for 20 weeks 8/64 |
0.1 mg/kg/day for 20 weeks 2/62 |
3.88 (0.86 to 17.53) |
| 0.05 mg/kg/day for 20 weeks 8/64 |
0.2 mg/kg/day for 20 weeks 0/65 |
17.26 (1.02 to 292.95)a | ||
| 0.1 mg/kg/day for 20 weeks 2/62 |
0.2 mg/kg/day for 20 weeks 0/65 |
5.24 (0.26 to 106.98) | ||
| 5. Dropout rates due to adverse effects (blurred vision) | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 0/25 |
> 0.5 mg/kg/day for 90 days 1/26 |
0.35 (0.01 to 8.12) |
| 6. Dropout rate due to adverse effects (raised serum triglycerides) | Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
Not estimable | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) | ||
| 7. Dropout rate due to adverse effects (raised liver function test) | Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
Not estimable | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) | ||
| 8. Dropout rates due to adverse effects (dry lips and dry skin) | Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 1/31 |
1.0 mg/kg/day for 12 weeks 3/27 |
0.29 (0.03 to 2.63) |
| 9. Dropout rates due to reasons not related to the intervention | Corlin 1984 | 0.05 mg/kg/day for 20 weeks 9/64 |
0.1 mg/kg/day for 20 weeks 3/62 |
2.91 (0.83 to 10.24) |
| 0.05 mg/kg/day for 20 weeks 9/64 |
0.2 mg/kg/day for 20 weeks 5/65 |
1.83 (0.65 to 5.16) | ||
|
0.1 mg/kg/day for 20 weeks 3/62 |
0.2 mg/kg/day for 20 weeks 5/65 |
0.63 (0.16 to 2.52) | ||
| Cumurcu 2009 | < 0.5 mg/kg/day for 90 days 1/25 |
> 0.5 mg/kg/day for 90 days 0/26 |
3.12 (0.13 to 73.06) | |
| 10. Dropout rates due to personal reasons | Lee 2011 | 0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 3/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 2/20 |
1.50 (0.28 to 8.04) |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 4/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 3/20 |
1.33 (0.34 to 5.21) | ||
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 4/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 2/20 |
2.00 (0.41 to 9.71) | ||
| 11. Dropout rates due to other reasons (not specified) | Van der Meeren 1983 | 0.5 mg/kg/day for 12 weeks 3/31 |
1.0 mg/kg/day for 12 weeks 0/27 |
6.13 (0.33 to 113.50) |
a Results that showed a statistically significant difference between interventions
* The interventions groups which had lower dropout rates in each comparison are in bold.
2. Oral isotretinoin versus oral isotretinoin plus topical agents
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
There were no serious adverse effects in either RCT which had addressed the comparison of oral isotretinoin versus oral isotretinoin plus topical agents (Dhir 2008 and Faghihi 2014) which involved 60 and 58 participants with moderate to severe acne, respectively. The quality of this evidence was low due to serious limitations of design in Faghihi 2014 (unclear risk of selection bias) and very serious limitations of design (high risk of performance, attrition and reporting bias) in the trial by Dhir 2008.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Two RCTs assessed this outcome and assessed different comparisons at different time points (Dhir 2008; Faghihi 2014).
Oral isotretinoin versus oral isotretinoin plus topical clindamycin 1% daytime plus adapalene 0.1% at bedtime (Dhir 2008): after 24 weeks, there was complete, excellent or good clearing of lesions in 100% (25/25) of the participants in the control group and 92% (23/25) of those participants on isotretinoin alone; hence, there was no clear difference between groups (RR 0.92, 95% CI 0.80 to 1.06; participants = 50; studies = 1). The definition used was as follows: 100% reduction in pretreatment score = complete clearing; from 75% to 99% = excellent; and from 50% to 74% = good. All participants had severe acne.
We downgraded the quality of evidence from this study from high to low (two levels) due to the very serious limitations of design (high risk of performance, attrition and reporting bias).
-
Oral isotretinoin versus oral isotretinoin plus 5% dapsone gel (Faghihi 2014) for moderate to severe acne: the investigators assessed the mean score in Global Acne Assessment Scale (GAAS), a 5‐point scale in which higher scores correlate with more severe acne, at baseline and at weeks 4, 8 and 12, and found the following results:
four weeks: participants in isotretinoin plus 5% dapsone gel group presented a 0.1 lower mean GAAS score but it was not statistically significant (MD 0.10, 95% CI ‐0.08 to 0.28; participants = 58; studies = 1);
eight weeks: participants on isotretinoin alone presented a 0.2 lower mean GAAS score, which means they achieved more clearance of acne lesions than participants in the isotretinoin plus 5% dapsone gel group (MD ‐0.20, 95% CI ‐0.35 to ‐0.05; participants = 58; studies = 1); and
12 weeks: participants on isotretinoin plus 5% dapsone gel had a 0.2 lower mean GAAS score, (MD 0.20, 95% CI 0.07 to 0.33; participants = 58; studies = 1).
The quality of evidence from Faghihi 2014 was downgraded from high to moderate (one level), for the effects measured at 4, 8 and 12 weeks, due to serious limitations of design in the study, more specifically unclear risk of selection bias related to both domains, random sequence generation and allocation concealment.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Two RCTs assessed this outcome (Dhir 2008; Faghihi 2014).
Oral isotretinoin versus oral isotretinoin plus topical clindamycin 1% daytime plus adapalene 0.1% at bedtime for severe acne: Dhir 2008 reported the frequency of each less serious adverse event for all randomised participants, without specifying the group. The exceptions were melasma‐like pigmentation, with a risk 66% higher among participants on oral isotretinoin alone but the confidence interval was wide and included 1 showing uncertainty (RR 1.66, 95% CI 0.44 to 6.24; participants = 50; studies = 1); and flare‐up during the first eight weeks of treatment, with an estimated risk 67% lower among participants in the oral isotretinoin alone group, though the confidence interval was also wide and included 1 (RR 0.33, 95% CI 0.10 to 1.09; participants =50; studies = 1). The quality of the evidence for 'melasma‐like pigmentation' was downgraded by three levels due to very serious limitations of design in the study (high risk of bias in three domains: performance, attrition, and reporting) and serious imprecision of the effect, which had a wide confidence interval. For the other less serious adverse effects, flare‐up at eight weeks of therapy, we downgraded the quality of evidence by two levels, from high‐ to low‐quality, and the reason was the very serious limitations of design in Dhir 2008, which we already referred to above.
Oral isotretinoin versus oral isotretinoin plus 5% dapsone gel for moderate to severe acne (Faghihi 2014): the frequency of less serious adverse effects was lower in the isotretinoin alone group (RR 0.17, 95% CI 0.06 to 0.51; participants = 58; studies = 1). We considered the quality of this evidence as moderate (a downgrade of one level) due to serious limitations of design in Faghihi 2014, more specifically unclear risk of selection bias related to both domains, random sequence generation and allocation concealment.
We also presented data of each less serious adverse event reported by all included RCTs in Table 4 and Table 7.
6. Less serious adverse effects from RCTs. Oral isotretinoin at different doses/schemes.
| Adverse effect (AE) outcomes | Study | – Oral isotretinoin in different doses/therapeutic regimens | Estimate of effect: risk ratio, 95% CI | |
|
Intervention Number of participants who experienced the AE/Number of analysed participants |
Control Number of participants who experienced the AE/Number of analysed participants |
|||
| 1. Clinical adverse effects ‐ mucocutaneous | ||||
| 1.1 Cheilitis (also chapped lips) | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks* 26/28 |
1 mg/kg/day, for 16 weeks 27/27 |
0.93 (0.82 to 1.05) |
|
20 mg (alternate day), for 16 weeks 26/29 |
1 mg/kg/day (alternate day), for 16 weeks 26/28 |
0.97 (0.82 to 1.13) | ||
|
20 mg (alternate day), for 16 weeks 26/29 |
1 mg/kg/day, for 16 weeks 27/27 |
0.90 (0.78 to 1.03) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 22/28 |
1 mg/kg/day, for 16 weeks 27/27 |
0.79 (0.65 to 0.97)a | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 22/28 |
1 mg/kg/day, (alternate day), for 16 weeks 26/28 |
0.85 (0.68 to 1.05) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 22/28 |
20 mg (alternate day), for 16 weeks 26/29 |
0.88 (0.70 to 1.10 ) | ||
| Corlin 1984 |
0.05 mg/kg/day for 20 weeks 34/64 |
0.1 mg/kg/day for 20 weeks 40/62 |
0.82 (0.61 to 1.11 ) | |
|
0.05 mg/kg/day for 20 weeks 34/64 |
0.2 mg/kg/day for 20 weeks 44/65 |
0.78 (0.59 to 1.04 ) | ||
|
0.1 mg/kg/day for 20 weeks 40/62 |
0.2 mg/kg/day for 20 weeks 44/65 |
0.95 (0.74 to 1.22 ) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 111/116 |
20 mg daily for 24 weeks 115/118 |
0.98 (0.93 to1.03) | |
| Jones 1983a |
0.1 mg/kg/day for 16 weeks 20/22 |
0.5 mg/kg/day for 16 weeks 29/30 |
0.94 (0.81 to 1.09) | |
|
0.1 mg/kg/day for 16 weeks 20/22 |
1.0 mg/kg/day for 16 weeks 22/24 |
0.99 (0.83 to 1.19) | ||
| 0.5 mg/kg/day for 16 weeks 29/30 |
1.0 mg/kg/day for 16 weeks 22/24 |
1.05 (0.92 to 1.21) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 35/46 |
0.5 mg/kg/day for 20 weeks 41/46 |
0.85 (0.71 to 1.03 ) | |
|
0.1 mg/kg/day for 20 weeks 35/46 |
1.0 mg/kg/day for 20 weeks 46/49 |
0.95 (0.84 to 1.07) | ||
|
0.5 mg/kg/day for 20 weeks 41/46 |
1.0 mg/kg/day for 20 weeks 46/49 |
0.95 (0.84 to 1.07) | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 22/31 |
1.0 mg/kg/day for 12 weeks 20/27 |
0.96 (0.70 to 1.32) | |
| 1.2 Dry lips | Corlin 1984 |
0.05 mg/kg/day for 20 weeks 60/64 |
0.1 mg/kg/day for 20 weeks 62/62 |
0.94 (0.87 to 1.01) |
|
0.05 mg/kg/day for 20 weeks 60/64 |
0.2 mg/kg/day for 20 weeks 65/65 |
0.94 (0.87 to 1.01) | ||
| 0.1 mg/kg/day for 20 weeks 62/62 |
0.2 mg/kg/day for 20 weeks 65/65 |
1.00 (0.97 to 1.03) | ||
| Van der Meeren 1983 | 0.5 mg/kg/day for 12 weeks 31/31 |
1.0 mg/kg/day for 12 weeks 27/27 |
1.00 (0.94 to 1.07) | |
| 1.3 Dry, chapped lips | Akman 2007 |
0.5 mg/kg/day first 10 days of each month for 6 months (intermittent) 16/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 18/19 |
0.77 (0.58 to 1.01) |
|
0.5 mg/kg/day first 10 days of each month for 6 months (intermittent) 16/22 |
0.5 mg/kg/day, for 6 months 19/19 |
0.74 (0.56 to 0.96)a | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 18/19 |
0.5 mg/kg/day, for 6 months 19/19 |
0.95 (0.82 to 1.09) | ||
| Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 11/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 15/20 |
0.73 (0.46 to 1.17) | |
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 7/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 11/20 |
0.64 (0.31 to 1.30) | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 7/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 15/20 |
0.47 (0.24 to 0.89 )a | ||
| 1.4 Dry skin | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 22/28 |
1 mg/kg/day, for 16 weeks 25/27 |
0.85 (0.68 to 1.06) |
| 20 mg (alternate day), for 16 weeks 23/29 |
1 mg/kg/day (alternate day), for 16 weeks 22/28 |
1.01 (0.77 to 1.32) | ||
|
20 mg (alternate day), for 16 weeks 23/29 |
1 mg/kg/day, for 16 weeks 25/27 |
0.86 (0.69 to 1.06) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 20/28 |
1 mg/kg/day, for 16 weeks 25/27 |
0.77 (0.60 to 1.00) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 20/28 |
1 mg/kg/day, (alternate day), for 16 weeks 22/28 |
0.91 (0.67 to 1.23) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 20/28 |
20 mg (alternate day), for 16 weeks 23/29 |
0.90 (0.67 to 1.21) | ||
| Akman 2007 |
0.5 mg/kg/day first 10 days of each month, for 6 months 13/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 13/19 |
0.86 (0.54 to 1.37) | |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 13/22 |
0.5 mg/kg/day, for 6 months 14/19 |
0.80 (0.52 to 1.24) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 13/19 |
0.5 mg/kg/day, for 6 months 14/19 |
0.93 (0.62 to 1.39) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 12/116 |
20 mg daily for 24 weeks 20/118 |
0.61 (0.31 to 1.19) | |
| Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 1/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 5/20 |
0.20 (0.03 to 1.56) | |
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 1/20 |
0.33 (0.01 to 7.72) | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 5/20 |
0.09 (0.01 to 1.54) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 26/46 |
0.5 mg/kg/day for 20 weeks 40/46 |
0.65 (0.49 to 0.86)a | |
|
0.1 mg/kg/day for 20 weeks 26/46 |
1.0 mg/kg/day for 20 weeks 40/49 |
0.69 (0.52 to 0.92)a | ||
| 0.5 mg/kg/day for 20 weeks 40/46 |
1.0 mg/kg/day for 20 weeks 40/49 |
1.07 (0.90 to 1.27) | ||
| 1.5 Peeling of fingertip skin | Akman 2007 |
0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 2/19 |
0.17 (0.01 to 3.41) |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 4/19 |
0.10 (0.01 to 1.69) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 2/19 |
0.5 mg/kg/day, for 6 months 4/19 |
0.50 (0.10 to 2.41) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 14/46 |
0.57 (0.27 to 1.23) | |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 24/49 |
0.36 (0.18 to 0.71)a | ||
|
0.5 mg/kg/day for 20 weeks 14/46 |
1.0 mg/kg/day for 20 weeks 24/49 |
0.62 (0.37 to 1.05) | ||
| 1.6 Rashes or facial redness (also facial dermatitis) | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 7/28 |
1 mg/kg/day, for 16 weeks 8/27 |
0.84 (0.36 to 2.01) |
|
20 mg (alternate day), for 16 weeks 5/29 |
1 mg/kg/day (alternate day), for 16 weeks 7/28 |
0.69 (0.25 to 1.92) | ||
|
20 mg (alternate day), for 16 weeks 5/29 |
1 mg/kg/day, for 16 weeks 8/27 |
0.58 (0.22 to 1.56) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 7/28 |
1 mg/kg/day, for 16 weeks 8/27 |
0.84 (0.36 to 2.01) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 7/28 |
1 mg/kg/day, (alternate day), for 16 weeks 7/28 |
1.00 (0.40 to 2.48) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 7/28 |
20 mg (alternate day), for 16 weeks 5/29 |
1.45 (0.52 to 4.03) | ||
| Akman 2007 |
0.5 mg/kg/day first 10 days of each month for 6 months 1/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 6/19 |
0.14 (0.02 to 1.09) | |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 1/22 |
0.5 mg/kg/day, for 6 months 9/19 |
0.10 (0.01 to 0.69)a | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 6/19 |
0.5 mg/kg/day, for 6 months 9/19 |
0.67 (0.30 to 1.50) | ||
| Corlin 1984 | 0.05 mg/kg/day for 20 weeks 18/64 |
0.1 mg/kg/day for 20 weeks 17/62 |
1.03 (0.58 to 1.80) | |
|
0.05 mg/kg/day for 20 weeks 18/64 |
0.2 mg/kg/day for 20 weeks 23/65 |
0.79 (0.48 to 1.33) | ||
|
0.1 mg/kg/day for 20 weeks 17/62 |
0.2 mg/kg/day for 20 weeks 23/65 |
0.77 (0.46 to 1.31) | ||
| Dhaked 2016 | 20 mg alternate days for 24 weeks 3/116 |
20 mg daily for 24 weeks 3/118 |
1.02 (0.21 to 4.94) | |
| Jones 1983a |
0.1 mg/kg/day for 16 weeks 11/22 |
0.5 mg/kg/day for 16 weeks 18/30 |
0.83 (0.50 to 1.39) | |
|
0.1 mg/kg/day for 16 weeks 11/22 |
1.0 mg/kg/day for 16 weeks 18/24 |
0.67 (0.41 to 1.07) | ||
|
0.5 mg/kg/day for 16 weeks 18/30 |
1.0 mg/kg/day for 16 weeks 18/24 |
0.80 (0.55 to 1.16) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 11/46 |
0.5 mg/kg/day for 20 weeks 20/46 |
0.55 (0.30 to 1.01) | |
|
0.1 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 33/49 |
0.36 (0.20 to 0.62)a | ||
|
0.5 mg/kg/day for 20 weeks 20/46 |
1.0 mg/kg/day for 20 weeks 33/49 |
0.65 (0.44 to 0.95)a | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 5/31 |
1.0 mg/kg/day for 12 weeks 8/27 |
0.54 (0.20 to 1.47) | |
| 1.7 Skin fragility (also increased risk of damage to skin) | Corlin 1984 |
0.05 mg/kg/day for 20 weeks 5/64 |
0.1 mg/kg/day for 20 weeks 6/62 |
0.81 (0.26 to 2.51) |
|
0.05 mg/kg/day for 20 weeks 5/64 |
0.2 mg/kg/day for 20 weeks 9/65 |
0.56 (0.20 to 1.59) | ||
|
0.1 mg/kg/day for 20 weeks 6/62 |
0.2 mg/kg/day for 20 weeks 9/65 |
0.70 (0.26 to 1.85) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 7/46 |
0.5 mg/kg/day for 20 weeks 14/46 |
0.50 (0.22 to 1.12) | |
|
0.1 mg/kg/day for 20 weeks 7/46 |
1.0 mg/kg/day for 20 weeks 19/49 |
0.39 (0.18 to 0.85)a | ||
|
0.5 mg/kg/day for 20 weeks 14/46 |
1.0 mg/kg/day for 20 weeks 19/49 |
0.78 (0.45 to 1.38) | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 3/31 |
1.0 mg/kg/day for 12 weeks 8/27 |
0.33 (0.10 to 1.11) | |
| 1.8 Scaling of skin (also rash, desquamation) | Jones 1983a | 0.1 mg/kg/day for 16 weeks 6/22 |
0.5 mg/kg/day for 16 weeks 8/30 |
1.02 (0.41 to 2.53) |
|
0.1 mg/kg/day for 16 weeks 6/22 |
1.0 mg/kg/day for 16 weeks 10/24 |
0.65 (0.29 to 1.50) | ||
|
0.5 mg/kg/day for 16 weeks 8/30 |
1.0 mg/kg/day for 16 weeks 10/24 |
0.64 (0.30 to 1.37) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 10/46 |
0.5 mg/kg/day for 20 weeks 12/46 |
0.83 (0.40 to 1.73) | |
|
0.1 mg/kg/day for 20 weeks 10/46 |
1.0 mg/kg/day for 20 weeks 22/49 |
0.48 (0.26 to 0.91)a | ||
|
0.5 mg/kg/day for 20 weeks 12/46 |
1.0 mg/kg/day for 20 weeks 22/49 |
0.58 (0.33 to 1.03) | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 19/31 |
1.0 mg/kg/day for 12 weeks 20/27 |
0.83 (0.58 to 1.18) | |
| 1.9 Repeated scaling of skin | Corlin 1984 |
0.05 mg/kg/day for 20 weeks 13/64 |
0.1 mg/kg/day for 20 weeks 18/62 |
0.70 (0.38, 1.30) |
|
0.05 mg/kg/day for 20 weeks 13/64 |
0.2 mg/kg/day for 20 weeks 29/65 |
0.46 (0.26 to 0.79)a | ||
|
0.1 mg/kg/day for 20 weeks 18/62 |
0.2 mg/kg/day for 20 weeks 29/65 |
0.65 (0.41 to 1.05) | ||
| 1.10 Skin atrophy | Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 0/31 |
1.0 mg/kg/day for 12 weeks 1/27 |
0.29 (0.01 to 6.88) |
| 1.11 Dry mouth | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 8/28 |
1 mg/kg/day, for 16 weeks 18/27 |
0.43 (0.23 to 0.82)a |
|
20 mg (alternate day), for 16 weeks 6/29 |
1 mg/kg/day (alternate day), for 16 weeks 8/28 |
0.72 (0.29 to 1.82) | ||
|
20 mg (alternate day), for 16 weeks 6/29 |
1 mg/kg/day, for 16 weeks 18/27 |
0.31 (0.15 to 0.66)a | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 5/28 |
1 mg/kg/day, for 16 weeks 18/27 |
0.27 (0.12 to 0.62)a | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 5/28 |
1 mg/kg/day, (alternate day), for 16 weeks 8/28 |
0.63 (0.23 to 1.68) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 5/28 |
20 mg (alternate day), for 16 weeks 6/29 |
0.86 (0.30 to 2.51) | ||
| Akman 2007 | 0.5 mg/kg/day first 10 days of each month for 6 months 2/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
1.73 (0.17 to 17.59) | |
|
0.5 mg/kg/day first 10 days of each month for 6 months 2/22 |
0.5 mg/kg/day, for 6 months 9/19 |
0.19 (0.05 to 0.78)a | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
0.5 mg/kg/day, for 6 months 9/19 |
0.11 (0.02 to 0.79)a | ||
| Corlin 1984 | 0.05 mg/kg/day for 20 weeks 14/64 |
0.1 mg/kg/day for 20 weeks 12/62 |
1.13 (0.57 to 2.25) | |
|
0.05 mg/kg/day for 20 weeks 14/64 |
0.2 mg/kg/day for 20 weeks 18/65 |
0.79 (0.43 to 1.45) | ||
|
0.1 mg/kg/day for 20 weeks 12/62 |
0.2 mg/kg/day for 20 weeks 18/65 |
0.70 (0.37 to 1.33) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 2/116 |
20 mg daily for 24 weeks 3/118 |
0.68 (0.11 to 3.98) | |
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 5/31 |
1.0 mg/kg/day for 12 weeks 12/27 |
0.36 (0.15, 0.90)a | |
| 1.12 Sore mouth | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 6/46 |
0.5 mg/kg/day for 20 weeks 6/46 |
1.00 (0.35 to 2.87) |
|
0.1 mg/kg/day for 20 weeks 6/46 |
1.0 mg/kg/day for 20 weeks 15/49 |
0.43 (0.18 to 1.00) | ||
|
0.5 mg/kg/day for 20 weeks 6/46 |
1.0 mg/kg/day for 20 weeks 15/49 |
0.43 (0.18 to 1.00) | ||
| 1.13 Dry nose | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
1 mg/kg/day, for 16 weeks 7/27 |
0.06 (0.00 to 1.07) |
| 20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
Not estimable | ||
|
20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day, for 16 weeks 7/27 |
0.06 (0.00 to 1.04) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
1 mg/kg/day, for 16 weeks 7/27 |
0.41 (0.12 to 1.44) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
1 mg/kg/day, (alternate day), for 16 weeks 0/28 |
7.00 (0.38 to 129.55) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
20 mg (alternate day), for 16 weeks 0/29 |
7.24 (0.39 to 134.12) | ||
| Corlin 1984 |
0.05 mg/kg/day for 20 weeks 17/64 |
0.1 mg/kg/day for 20 weeks 18/62 |
0.91 (0.52 to 1.61) | |
|
0.05 mg/kg/day for 20 weeks 17/64 |
0.2 mg/kg/day for 20 weeks 25/65 |
0.69 (0.41 to 1.15) | ||
|
0.1 mg/kg/day for 20 weeks 18/62 |
0.2 mg/kg/day for 20 weeks 25/65 |
0.75 (0.46 to 1.24) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 4/118 |
0.25 (0.03 to 2.24) | |
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 23/46 |
0.5 mg/kg/day for 20 weeks 37/46 |
0.62 (0.45 to 0.86)a | |
|
0.1 mg/kg/day for 20 weeks 23/46 |
1.0 mg/kg/day for 20 weeks 38/49 |
0.64 (0.47 to 0.89)a | ||
| 0.5 mg/kg/day for 20 weeks 37/46 |
1.0 mg/kg/day for 20 weeks 38/49 |
1.04 (0.84 to 1.28) | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 10/31 |
1.0 mg/kg/day for 12 weeks 17/27 |
0.51 (0.29 to 0.92)a | |
| 1.14 Epistaxis (also nasal bleeding) | Akman 2007 | 0.5 mg/kg/day first 10 days of each month for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
Not estimable |
|
0.5 mg/kg/day first 10 days of each month for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 2/19 |
0.17 (0.01 to 3.41) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
0.5 mg/kg/day, for 6 months 2/19 |
0.20 (0.01 to 3.91) | ||
| Jones 1983a |
0.1 mg/kg/day for 16 weeks 6/22 |
0.5 mg/kg/day for 16 weeks 9/30 |
0.91 (0.38 to 2.18) | |
|
0.1 mg/kg/day for 16 weeks 6/22 |
1.0 mg/kg/day for 16 weeks 11/24 |
0.60 (0.26 to 1.34) | ||
|
0.5 mg/kg/day for 16 weeks 9/30 |
1.0 mg/kg/day for 16 weeks 11/24 |
0.65 (0.33 to 1.32) | ||
| Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 3/20 |
0.14 (0.01 to 2.60) | |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
Not estimable | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 3/20 |
0.14 (0.01 to 2.60) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 13/46 |
0.62 (0.28 to 1.34) | |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 26/49 |
0.33 (0.17 to 0.65)a | ||
|
0.5 mg/kg/day for 20 weeks 13/46 |
1.0 mg/kg/day for 20 weeks 26/49 |
0.53 (0.31 to 0.91)a | ||
| 1.15 Dryness of other mucosal tissues | Akman 2007 |
0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
0.29 (0.01 to 6.72) |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 4/19 |
0.10 (0.01 to 1.69) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
0.5 mg/kg/day, for 6 months 4/19 |
0.25 (0.03 to 2.04) | ||
| 1.16 Dry eyes | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
1 mg/kg/day, for 16 weeks 10/27 |
0.05 (0.00 to 0.75)a |
| 20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
Not estimable | ||
|
20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day, for 16 weeks 10/27 |
0.04 (0.00 to 0.72)a | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
1 mg/kg/day, for 16 weeks 10/27 |
0.29 (0.09 to 0.94)a | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
1 mg/kg/day, (alternate day), for 16 weeks 0/28 |
7.00 (0.38 to 129.55) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 3/28 |
20 mg (alternate day), for 16 weeks 0/29 |
7.24 (0.39 to 134.12) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 2/116 |
20 mg daily for 24 weeks 9/118 |
0.23 (0.05 to 1.02) | |
| 1.17 Dry or irritated eyes | Akman 2007 | 0.5 mg/kg/day first 10 days of each month, for 6 months 2/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
1.73 (0.17 to 17.59) |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 2/22 |
0.5 mg/kg/day, for 6 months 3/19 |
0.58 (0.11 to 3.09) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 1/19 |
0.5 mg/kg/day, for 6 months 3/19 |
0.33 (0.04 to 2.93) | ||
| 1.18 Irritation of eyes | Strauss 1984 |
0.1 mg/kg/day for 20 weeks 20/46 |
0.5 mg/kg/day for 20 weeks 23/46 |
0.87 (0.56 to 1.35) |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 26/49 |
0.33 (0.17 to 0.65)a | ||
|
0.5 mg/kg/day for 20 weeks 23/46 |
1.0 mg/kg/day for 20 weeks 28/49 |
0.88 (0.60 to 1.28) | ||
| 1.19 Conjunctivitis | Corlin 1984 | 0.05 mg/kg/day for 20 weeks 6/64 |
0.1 mg/kg/day for 20 weeks 5/62 |
1.16 (0.37 to 3.61) |
| 0.05 mg/kg/day for 20 weeks 6/64 |
0.2 mg/kg/day for 20 weeks 5/65 |
1.22 (0.39 to 3.79) | ||
| 0.1 mg/kg/day for 20 weeks 5/62 |
0.2 mg/kg/day for 20 weeks 5/65 |
1.05 (0.32 to 3.45) | ||
| Jones 1983a |
0.1 mg/kg/day for 16 weeks 0/22 |
0.5 mg/kg/day for 16 weeks 4/30 |
0.15 (0.01 to 2.65) | |
|
0.1 mg/kg/day for 16 weeks 0/22 |
1.0 mg/kg/day for 16 weeks 5/24 |
0.10 (0.01 to 1.69) | ||
|
0.5 mg/kg/day for 16 weeks 4/30 |
1.0 mg/kg/day for 16 weeks 5/24 |
0.64 (0.19 to 2.13) | ||
| 1.20 Blepharoconjunctivitis | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 2/25 |
> 0.5 mg/kg/day for 90 days 5/26 |
0.42 (0.09 to 1.95) |
| 1.21 Severe blepharoconjunctivitis | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 0/25 |
> 0.5 mg/kg/day for 90 days 1/26 |
0.35 (0.01 to 8.12) |
| 1.22 Blurred vision | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 0/25 |
> 0.5 mg/kg/day for 90 days 1/26 |
0.35 (0.01 to 8.12) |
| 1.23 Contact lens intolerance | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 0/25 |
> 0.5 mg/kg/day for 90 days 1/26 |
0.35 (0.01 to 8.12) |
| 1.24 Pruritus (also itching) | Akman 2007 |
0.5 mg/kg/day first 10 days of each month, for 6 months 3/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 3/19 |
0.86 (0.20 to 3.79) |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 3/22 |
0.5 mg/kg/day, for 6 months 6/19 |
0.43 (0.12 to 1.50) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 3/19 |
0.5 mg/kg/day, for 6 months 6/19 |
0.50 (0.15 to 1.71) | ||
| Corlin 1984 | 0.05 mg/kg/day for 20 weeks 10/64 |
0.1 mg/kg/day for 20 weeks 8/62 |
1.21 (0.51 to 2.87) | |
| 0.05 mg/kg/day for 20 weeks 10/64 |
0.2 mg/kg/day for 20 weeks 8/65 |
1.27 (0.54 to 3.01) | ||
| 0.1 mg/kg/day for 20 weeks 8/62 |
0.2 mg/kg/day for 20 weeks 8/65 |
1.05 (0.42 to 2.62) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 5/116 |
20 mg daily for 24 weeks 11/118 |
0.46 (0.16 to 1.29) | |
| Strauss 1984 | 0.1 mg/kg/day for 20 weeks 22/46 |
0.5 mg/kg/day for 20 weeks 17/46 |
1.29 (0.80 to 2.10) | |
|
0.1 mg/kg/day for 20 weeks 22/46 |
1.0 mg/kg/day for 20 weeks 33/49 |
0.71 (0.50 to 1.02) | ||
|
0.5 mg/kg/day for 20 weeks 17/46 |
1.0 mg/kg/day for 20 weeks 33/49 |
0.55 (0.36 to 0.84)a | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 10/31 |
1.0 mg/kg/day for 12 weeks 15/27 |
0.58 (0.31, 1.07) | |
| 1.25 Hair loss | Akman 2007 | 0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
Not estimable |
| 0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| 0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| Corlin 1984 | 0.05 mg/kg/day for 20 weeks 7/64 |
0.1 mg/kg/day for 20 weeks 6/62 |
1.13 (0.40 to 3.18) | |
| 0.05 mg/kg/day for 20 weeks 7/64 |
0.2 mg/kg/day for 20 weeks 7/65 |
1.02 (0.38, 2.73) | ||
|
0.1 mg/kg/day for 20 weeks 6/62 |
0.2 mg/kg/day for 20 weeks 7/65 |
0.90 (0.32 to 2.53) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 3/116 |
20 mg daily for 24 weeks 7/118 |
0.44 (0.11 to 1.64) | |
| Strauss 1984 | 0.1 mg/kg/day for 20 weeks 12/46 |
0.5 mg/kg/day for 20 weeks 12/46 |
1.00 (0.50 to 1.99) | |
|
0.1 mg/kg/day for 20 weeks 12/46 |
1.0 mg/kg/day for 20 weeks 17/49 |
0.75 (0.40 to 1.40) | ||
|
0.5 mg/kg/day for 20 weeks 12/46 |
1.0 mg/kg/day for 20 weeks 17/49 |
0.75 (0.40 to 1.40) | ||
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 0/31 |
1.0 mg/kg/day for 12 weeks 3/27 |
0.13 (0.01 to 2.32) | |
| 1.26 Sunburn | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 7/46 |
0.5 mg/kg/day for 20 weeks 4/46 |
1.75 (0.55 to 5.57) |
| 0.1 mg/kg/day for 20 weeks 7/46 |
1.0 mg/kg/day for 20 weeks 5/49 |
1.49 (0.51 to 4.37) | ||
|
0.5 mg/kg/day for 20 weeks 4/46 |
1.0 mg/kg/day for 20 weeks 5/49 |
0.85 (0.24 to 2.98) | ||
| 1.27 Oral aphthous | Dhaked 2016 |
20 mg alternate days for 24 weeks 0/116 |
20 mg daily for 24 weeks 1/118 |
0.34 (0.01 to 8.24) |
| 1.28 Urticaria | Dhaked 2016 |
20 mg alternate days for 24 weeks 3/116 |
20 mg daily for 24 weeks 4/118 |
0.76 (0.17 to 3.33) |
| 1.29 Pigmentation of face | Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 0/118 |
3.05 (0.12 to 74.14) |
| 1.30 Dermographism | Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 0/118 |
3.05 (0.12 to 74.14) |
| 2. Clinical adverse effects (other than mucocutaneous) | ||||
| 2.1 Headache | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 10/46 |
0.5 mg/kg/day for 20 weeks 5/46 |
2.00 (0.74 to 5.40) |
|
0.1 mg/kg/day for 20 weeks 10/46 |
1.0 mg/kg/day for 20 weeks 12/49 |
0.89 (0.43 to 1.85) | ||
|
0.5 mg/kg/day for 20 weeks 5/46 |
1.0 mg/kg/day for 20 weeks 12/49 |
0.44 (0.17 to 1.16) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 2/118 |
0.51 (0.05 to 5.53) | |
| 2.2 Bone/joint aches and pains | Akman 2007 | 0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
Not estimable |
| 0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| 0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 11/46 |
0.73 (0.32 to 1.64) | |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 15/49 |
0.57 (0.27 to 1.21) | ||
|
0.5 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 15/49 |
0.78 (0.40 to 1.52) | ||
| 2.3 Arthralgia | Jones 1983a | 0.1 mg/kg/day for 16 weeks 4/22 |
0.5 mg/kg/day for 16 weeks 5/30 |
1.09 (0.33 to 3.60) |
|
0.1 mg/kg/day for 16 weeks 4/22 |
1.0 mg/kg/day for 16 weeks 7/24 |
0.62 (0.21 to 1.84) | ||
|
0.5 mg/kg/day for 16 weeks 5/30 |
1.0 mg/kg/day for 16 weeks 7/24 |
0.57 (0.21 to 1.58) | ||
| Dhaked 2016 |
20 mg alternate days for 24 weeks 0/116 |
20 mg daily for 24 weeks 1/118 |
0.34 (0.01 to 8.24) | |
| 2.4 Muscular cramps or pains | Akman 2007 | 0.5 mg/kg/day first 10 days of each month for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
Not estimable |
| 0.5 mg/kg/day first 10 days of each month for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| 0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
0.5 mg/kg/day, for 6 months 0/19 |
Not estimable | ||
| 2.5 Muscle cramps | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 4/46 |
2.00 (0.65 to 6.18) |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 11/49 |
0.77 (0.34 to 1.75) | ||
|
0.5 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 15/49 |
0.39 (0.13 to 1.13) | ||
| 2.6 Muscle pain | Corlin 1984 |
0.05 mg/kg/day for 20 weeks 6/64 |
0.1 mg/kg/day for 20 weeks 6/62 |
0.97 (0.33 to 2.84) |
| 0.05 mg/kg/day for 20 weeks 6/64 |
0.2 mg/kg/day for 20 weeks 5/65 |
1.22 (0.39 to 3.79) | ||
| 0.1 mg/kg/day for 20 weeks 6/62 |
0.2 mg/kg/day for 20 weeks 5/65 |
1.26 (0.40 to 3.91) | ||
| Dhaked 2016 | 20 mg alternate days for 24 weeks 2/116 |
20 mg daily for 24 weeks 2/118 |
1.02 (0.14 to 7.10) | |
| Van der Meeren 1983 |
0.5 mg/kg/day for 12 weeks 1/31 |
1.0 mg/kg/day for 12 weeks 1/27 |
0.87 (0.06 to 13.27) | |
| 2.7 Fatigue | Akman 2007 | 0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
Not estimable |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 0/22 |
0.5 mg/kg/day, for 6 months 1/19 |
0.29 (0.01 to 6.72) | ||
|
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 0/19 |
0.5 mg/kg/day, for 6 months 1/19 |
0.33 (0.01 to 7.70) | ||
| Strauss 1984 | 0.1 mg/kg/day for 20 weeks 12/46 |
0.5 mg/kg/day for 20 weeks 8/46 |
1.50 (0.68 to 3.32) | |
|
0.1 mg/kg/day for 20 weeks 12/46 |
1.0 mg/kg/day for 20 weeks 19/49 |
0.67 (0.37 to 1.23) | ||
|
0.5 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 19/49 |
0.45 (0.22 to 0.92)a | ||
| 2.8 Excessive thirst | Akman 2007 |
0.5 mg/kg/day first 10 days of each month for 6 months 2/22 |
0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 4/19 |
0.43 (0.09 to 2.10) |
|
0.5 mg/kg/day first 10 days of each month, for 6 months 2/22 |
0.5 mg/kg/day, for 6 months 3/19 |
0.58 (0.11 to 3.09) | ||
| 0.5 mg/kg/day for 1 month, followed by 0.5 mg/kg/day first 10 days of each month for 5 months 4/19 |
0.5 mg/kg/day, for 6 months 3/19 |
1.33 (0.34, 5.17) | ||
| Strauss 1984 | 0.1 mg/kg/day for 20 weeks 14/46 |
0.5 mg/kg/day for 20 weeks 11/46 |
1.27 (0.65 to 2.50) | |
| 0.1 mg/kg/day for 20 weeks 14/46 |
1.0 mg/kg/day for 20 weeks 11/49 |
1.36 (0.69 to 2.67) | ||
| 0.5 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 11/49 |
1.07 (0.51, 2.22) | ||
| 2.9 Malaise | Jones 1983a |
0.1 mg/kg/day for 16 weeks 1/22 |
0.5 mg/kg/day for 16 weeks 2/30 |
0.68 (0.07 to 7.05) |
|
0.1 mg/kg/day for 16 weeks 1/22 |
1.0 mg/kg/day for 16 weeks 4/24 |
0.27 (0.03 to 2.26) | ||
|
0.5 mg/kg/day for 16 weeks 2/30 |
1.0 mg/kg/day for 16 weeks 4/24 |
0.40 (0.08 to 2.00) | ||
| 2.10 Frequent mood changes | Kapadia 2005 |
20 mg/daily for 24 weeks 0/30 |
40 mg/daily 3/30 |
0.14 (0.01 to 2.65) |
| 2.11 Insomnia | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 12/46 |
0.5 mg/kg/day for 20 weeks 6/46 |
2.00 (0.82, 4.87) |
|
0.1 mg/kg/day for 20 weeks 12/46 |
1.0 mg/kg/day for 20 weeks 14/49 |
0.91 (0.47, 1.76) | ||
|
0.5 mg/kg/day for 20 weeks 6/46 |
1.0 mg/kg/day for 20 weeks 14/49 |
0.46 (0.19 to 1.09) | ||
| 2.12 Nausea | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 14/46 |
0.5 mg/kg/day for 20 weeks 4/46 |
3.50 (1.25 to 9.84) |
| 0.1 mg/kg/day for 20 weeks 14/46 |
1.0 mg/kg/day for 20 weeks 6/49 |
2.49 (1.04 to 5.92) | ||
|
0.5 mg/kg/day for 20 weeks 4/46 |
1.0 mg/kg/day for 20 weeks 6/49 |
0.71 (0.21 to 2.36) | ||
| 2.13 Decreased appetite | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 9/46 |
0.5 mg/kg/day for 20 weeks 6/46 |
1.50 (0.58 to 3.87) |
| 0.1 mg/kg/day for 20 weeks 9/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
1.37 (0.56 to 3.38) | ||
|
0.5 mg/kg/day for 20 weeks 6/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
0.91 (0.33 to 2.52) | ||
| 2.14 Increased appetite | Strauss 1984 |
0.1 mg/kg/day for 20 weeks 1/46 |
0.5 mg/kg/day for 20 weeks 3/46 |
0.33 (0.04 to 3.09) |
|
0.1 mg/kg/day for 20 weeks 1/46 |
1.0 mg/kg/day for 20 weeks 2/49 |
0.53 (0.05 to 5.68) | ||
| 0.5 mg/kg/day for 20 weeks 3/46 |
1.0 mg/kg/day for 20 weeks 2/49 |
1.60 (0.28 to 9.13) | ||
| 2.15 Abdominal pain | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
4.00 (0.90 to 17.83) |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.95 (0.40 to 2.24) | ||
|
0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.24 (0.05 to 1.04) | ||
| 2.16 Eye pain | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 5/46 |
1.60 (0.57 to 4.53) |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.95 (0.40 to 2.24) | ||
|
0.5 mg/kg/day for 20 weeks 5/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.59 (0.21 to 1.64) | ||
| 2.17 Double vision | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 3/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
1.50 (0.26 to 8.56) |
|
0.1 mg/kg/day for 20 weeks 3/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
0.80 (0.19 to 3.38) | ||
|
0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
0.53 (0.10 to 2.77) | ||
| 2.18 Fever | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 4/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
2.00 (0.39 to 10.39) |
| 0.1 mg/kg/day for 20 weeks 4/46 |
1.0 mg/kg/day for 20 weeks 1/49 |
4.26 (0.49 to 36.73) | ||
| 0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 1/49 |
2.13 (0.20 to 22.71) | ||
| 2.19 Bruising | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 2/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
1.00 (0.15 to 6.80) |
|
0.1 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
0.53 (0.10 to 2.77) | ||
|
0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
0.53 (0.10 to 2.77) | ||
| 2.20 Forgetfulness | Dhaked 2016 |
20 mg alternate days for 24 weeks 0/116 |
20 mg daily for 24 weeks 1/118 |
0.34 (0.01 to 8.24) |
| 2.21 Menstrual irregularities | Dhaked 2016 | 20 mg alternate days for 24 weeks 2/25 |
20 mg daily for 24 weeks 1/20 |
1.60 (0.16 to 16.4) |
| 3. Serum laboratory adverse effects | ||||
| 3.1 Raised lipid blood levels | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 1/28 |
1 mg/kg/day, for 16 weeks 2/27 |
0.48 (0.05 to 5.01) |
|
20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day (alternate day), for 16 weeks 1/28 |
0.32 (0.01 to 7.59) | ||
|
20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day, for 16 weeks 2/27 |
0.19 (0.01 to 3.72) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
1 mg/kg/day, for 16 weeks 2/27 |
0.19 (0.01 to 3.85) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
1 mg/kg/day, (alternate day), for 16 weeks 1/28 |
0.33 (0.01 to 7.85) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
20 mg (alternate day), for 16 weeks 0/29 |
Not estimable | ||
| 3.2 Abnormal lipid profile | Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 4/118 |
0.25 (0.03 to 2.24) |
| 3.3 Increased triglycerides | Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
Not estimable | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) | ||
| Strauss 1984 |
0.1 mg/kg/day for 20 weeks 2/46 |
0.5 mg/kg/day for 20 weeks 5/46 |
0.40 (0.08 to 1.96) | |
|
0.1 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 12/49 |
0.18 (0.04 to 0.75)a | ||
|
0.5 mg/kg/day for 20 weeks 5/46 |
1.0 mg/kg/day for 20 weeks 12/49 |
0.44 (0.17 to 1.16) | ||
| 3.4 Decreased HDL (high‐density lipoprotein) levels | Strauss 1984 |
0.1 mg/kg/day for 20 weeks 6/46 |
0.5 mg/kg/day for 20 weeks 9/46 |
0.67 (0.26 to 1.72) |
|
0.1 mg/kg/day for 20 weeks 6/46 |
1.0 mg/kg/day for 20 weeks 8/49 |
0.80 (0.30 to 2.13) | ||
| 0.5 mg/kg/day for 20 weeks 9/46 |
1.0 mg/kg/day for 20 weeks 8/49 |
1.20 (0.51 to 2.84) | ||
| 3.5 Liver enzymes elevation | Agarwal 2011 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
1 mg/kg/day, for 16 weeks 1/27 |
0.32 (0.01 to 7.57) |
| 20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day (alternate day), for 16 weeks 0/28 |
Not estimable | ||
|
20 mg (alternate day), for 16 weeks 0/29 |
1 mg/kg/day, for 16 weeks 1/27 |
0.31 (0.01 to 7.33) | ||
|
1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
1 mg/kg/day, for 16 weeks 1/27 |
0.32 (0.01 to 7.57) | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
1 mg/kg/day, (alternate day), for 16 weeks 0/28 |
Not estimable | ||
| 1 mg/kg/day for 1 week/4 weeks (intermittent), for 16 weeks 0/28 |
20 mg (alternate day), for 16 weeks 0/29 |
Not estimable | ||
| Lee 2011 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) | |
| 0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.25 to 0.4 mg/kg/day for 24 weeks (continuous low dose) 0/20 |
Not estimable | ||
|
0.5 to 0.7 mg/kg/day, first 1 week in every 4 weeks for 24 weeks (intermittent conventional dose) 0/20 |
0.5 to 0.7 mg/kg/day for 24 weeks (continuous conventional dose) 1/20 |
0.33 (0.01 to 7.72) | ||
| 3.6 Abnormal liver function test | Dhaked 2016 |
20 mg alternate days for 24 weeks 1/116 |
20 mg daily for 24 weeks 3/118 |
0.34 (0.03 to 3.21) |
| 3.7 Increased aspartate aminotransferase levels | Jones 1983a |
0.1 mg/kg/day for 16 weeks 4/22 |
0.5 mg/kg/day for 16 weeks 13/30 |
0.42 (0.16 to 1.11) |
|
0.1 mg/kg/day for 16 weeks 4/22 |
1.0 mg/kg/day for 16 weeks 19/24 |
0.23 (0.09 to 0.57)a | ||
|
0.5 mg/kg/day for 16 weeks 13/30 |
1.0 mg/kg/day for 16 weeks 19/24 |
0.55 (0.35, 0.87)a | ||
| Strauss 1984 | 0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 7/46 |
1.14 (0.45 to 2.89) | |
|
0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.95 (0.40 to 2.24) | ||
|
0.5 mg/kg/day for 20 weeks 7/46 |
1.0 mg/kg/day for 20 weeks 9/49 |
0.83 (0.34 to 2.04) | ||
| 3.8 Increased LDH (lactate dehydrogenase) | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 11/46 |
0.5 mg/kg/day for 20 weeks 11/46 |
1.00 (0.48, 2.07) |
| 0.1 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 10/49 |
1.17 (0.55, 2.50) | ||
| 0.5 mg/kg/day for 20 weeks 11/46 |
1.0 mg/kg/day for 20 weeks 10/49 |
1.17 (0.55 to 2.50) | ||
| 3.9 Increased total protein levels | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 8/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
4.00 (0.90 to 17.83) |
| 0.1 mg/kg/day for 20 weeks 8/46 |
1.0 mg/kg/day for 20 weeks 5/49 |
1.70 (0.60 to 4.83) | ||
|
0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 5/49 |
0.43 (0.09 to 2.09) | ||
| 3.10 Increased platelets | Strauss 1984 |
0.1 mg/kg/day for 20 weeks 2/46 |
0.5 mg/kg/day for 20 weeks 5/46 |
0.40 (0.08 to 1.96) |
|
0.1 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
0.53 (0.10 to 2.77) | ||
| 0.5 mg/kg/day for 20 weeks 5/46 |
1.0 mg/kg/day for 20 weeks 4/49 |
1.33 (0.38 to 4.66) | ||
| 4. Urinary laboratory adverse effects | ||||
| 4.1 Increased specific gravity | Strauss 1984 |
0.1 mg/kg/day for 20 weeks 3/46 |
0.5 mg/kg/day for 20 weeks 5/46 |
0.60 (0.15 to 2.37) |
|
0.1 mg/kg/day for 20 weeks 3/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
0.46 (0.13 to 1.66) | ||
|
0.5 mg/kg/day for 20 weeks 5/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
0.76 (0.26 to 2.23) | ||
| 4.2 Increased white blood count | Strauss 1984 | 0.1 mg/kg/day for 20 weeks 2/46 |
0.5 mg/kg/day for 20 weeks 2/46 |
1.00 (0.15, 6.80) |
|
0.1 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
0.30 (0.07 to 1.39) | ||
|
0.5 mg/kg/day for 20 weeks 2/46 |
1.0 mg/kg/day for 20 weeks 7/49 |
0.30 (0.07 to 1.39) | ||
| 5. Bacteriological laboratory adverse effects | ||||
| 5.1 Staphylococcus aureus colonization of the conjunctiva day 45 | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 4/25 |
> 0.5 mg/kg/day for 90 days 10/26 |
0.42 (0.15 to 1.16) |
| 5.2 Staphylococcus aureus colonization of the conjunctiva day 90 | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 5/25 |
> 0.5 mg/kg/day for 90 days 10/26 |
0.52 (0.21 to 1.31) |
| 5.3 Staphylococcus aureus colonization of the conjunctiva follow‐up | Cumurcu 2009 |
< 0.5 mg/kg/day for 90 days 3/25 |
> 0.5 mg/kg/day for 90 days 4/26 |
0.78 (0.19 to 3.14) |
aResults that showed a statistically significant difference between interventions
bLDH: lactate dehydrogenase
*The intervention groups which had lower less serious adverse event rates in each comparison are in bold.
Secondary outcome: Dropout rates
Two RCTs assessed this outcome in participants with moderate and severe acne. We pooled data from these studies in meta‐analysis for overall dropout rates (Dhir 2008; Faghihi 2014). There was no difference in effect between groups for this outcome (RR 1.00, 95% CI 0.32 to 3.10; participants = 118; studies = 2; I² = 0%) (Analysis 2.1). We downgraded the quality of evidence by three levels to very low due to very serious limitations of design in the involved studies (Dhir 2008 had high risk of performance, attrition and reporting bias; Faghihi 2014 presented unclear risk of selection bias) and serious imprecision related to the wide confidence interval of the effect.
2.1. Analysis.
Comparison 2 Oral isotretinoin versus oral isotretinoin plus any topical agent, Outcome 1 Dropout rates.
Additionally, we presented the data concerning dropout rates reported for all included RCTs in Table 5 and Table 6.
3. Oral isotretinoin versus 0.1% tretinoin cream plus 5% benzoyl peroxide gel
We included one RCT on this comparison (Leheta 2011).
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
According to the report (conference abstract) of Leheta 2011, oral isotretinoin (0.5 mg/kg/day for 2 weeks, followed by 1 mg/kg/day until the end of therapy) was equivalent to 0.1% tretinoin cream each evening plus 5% benzoyl peroxide gel each morning to the face as regards improving acne severity score at the end of therapy in participants with mild to moderate acne. The authors presented a P value of 0.40, and no estimate of effect was reported in the text.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
There were no available data for this outcome.
Secondary outcome: Dropout rates
There were no available data for this outcome.
4. Oral isotretinoin versus chemical peeling with trichloroacetic acid (TCA) 25%
Leheta 2011 was the sole study which addressed this comparison.
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
In Leheta 2011, authors stated there was no difference between oral isotretinoin (0.5 mg/kg/day for 2 weeks, followed by 1 mg/kg/day until the end of therapy) versus chemical peeling with trichloroacetic acid (TCA) 25% (every two weeks for eight sessions; and monthly during follow‐up) regarding acne severity score at the end of therapy in participants with mild to moderate acne. The report did not cite any figures for the estimate of effect or additional details except for a P value of 0.40.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
There were no available data for this outcome.
Secondary outcome: Dropout rates
There were no available data for this outcome.
5. Oral isotretinoin versus oral isotretinoin plus oral antibiotic
We found two studies which analysed the comparison: De 2011 and Jones 1983b.
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome in De 2011. The trial by Jones 1983b assessed the outcome but did not provide any specific numerical data.
Primary outcome: Frequency of serious adverse effects
There were no serious adverse effects in De 2011 (n = 41) and Jones 1983b (n = 90). The quality of the evidence was low due to very serious limitations of design in both studies: unclear risk of selection, performance and detection bias and high risk of attrition bias in De 2011; unclear risk of selection bias and high risk of reporting bias in Jones 1983b.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
In the study by De 2011, which had focused on severe acne, participants in Group A (n = 21) received low dose isotretinoin (0.3 mg/kg/day) plus azithromycin pulse (500 mg daily on three consecutive days fortnightly) and had a 50% decrease in visual analogue scale (VAS) severity in a mean period of 3.37 months. Participants from Group B (n = 20) used standard‐dose isotretinoin (0.5 mg/kg/day) in a total cumulative dose of 120 mg/kg and had a 50% decrease in VAS with a mean period of 3.82 months. No P value or standard deviations were provided. Participants in both groups received interventions over eight months.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Only Jones 1983b assessed the outcome but did not provide any specific numerical data.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Both studies for the comparison, De 2011 and Jones 1983b, did not provide adequate data related to frequency of less serious adverse effects, despite having measured this outcome.
Secondary outcome: Dropout rates
From both studies in the comparison, only Jones 1983b assessed the outcome but did not provide any specific numerical data. The study involved 90 participants with moderate and severe acne and compared isotretinoin 0.5 mg/kg/day for 16 weeks (Group A, n = not provided) with erythromycin 1 g/kg/day for 24 weeks (Group B, n = not provided) and isotretinoin 0.5 mg/kg/day for 16 weeks plus erythromycin 1 g/kg/day for 24 weeks (Group C, n = not provided).
6. Oral isotretinoin versus oral azithromycin
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
Wahab 2008 was the only included RCT in this comparison and no serious adverse effects were detected among 60 participants, who had moderate to severe acne. The quality of the evidence was low due to very serious limitations of design in the study, more specifically high risk of selection, performance, and detection bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
One trial assessed this outcome and compared oral isotretinoin 0.5 to 1.0 mg/kg/daily for five months versus azithromycin 500 mg 3 days a week during three months for moderate to severe acne (Wahab 2008). The proportion of participants who presented an excellent (complete clearance) or good (> 75% clearing) improvement of acne severity at the end of therapy was 93% higher in the isotretinoin group (RR 1.93, 95% CI 1.34 to 2.78; participants = 60; studies= 1). We considered the quality of evidence as being low due to the very serious limitations of design in the study (high risk of performance and detection bias, besides the absence of allocation concealment). The proportion of participants with moderate acne who presented an excellent (complete clearance) or good (> 75% clearing) response was again higher (75%) in the isotretinoin group (RR 1.75, 95% CI 1.24 to 2.48; participants = 60; studies= 1). The quality of the evidence was low due to very serious limitations of design in Wahab 2008. Among those participants with severe acne, oral isotretinoin was more than four times more effective than azithromycin in promoting an excellent or good clinical response but the confidence interval was wide and included 1 showing uncertainty (RR 4.50, 95% CI 0.77 to 26.2; participants = 60; studies= 1). We downgraded the quality of evidence related to this last effect estimate by three levels, from high to very low due to very serious limitations of design and serious imprecision of the effect (wide confidence interval).
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Wahab 2008 assessed this outcome and detailed the proportion of participants who presented with an increase of serum lipids only in the isotretinoin group (3/30); the study also reported mild nausea and abdominal discomfort (3/30) only for the azithromycin group.
Secondary outcome: Dropout rates
There were no dropouts in the Wahab 2008 study.
7. Oral isotretinoin versus oral erythromycin
We found one RCT (Jones 1983b) for the comparison.
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
Jones 1983b assessed the outcome but did not provide any specific numerical data.
Primary outcome: Frequency of serious adverse effects
There was no serious adverse event in the study by Jones 1983b, which involved 90 participants with moderate and severe acne and compared isotretinoin 0.5 mg/kg/day for 16 weeks (Group A, n = not provided) with erythromycin 1 g/kg/day for 24 weeks (Group B, n = not provided) and isotretinoin 0.5 mg/kg/day for 16 weeks plus erythromycin 1 g/kg/day for 24 weeks (Group C, n = not provided). The quality of this evidence was low due to very serious limitations of design, more specifically unclear risk of selection bias and high risk of reporting bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Jones 1983b assessed the outcome but did not provide any specific numerical data.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Jones 1983b assessed the outcome but did not provide any specific numerical data.
Secondary outcome: Dropout rates
Jones 1983b did not report detailed data related to dropouts from each intervention group. Authors just cited the withdrawal of four participants (from a total of 90) due to side effects.
8. Oral isotretinoin versus oral minocycline
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
There was no report of any serious adverse event among participants of the included trial for this comparison (Pigatto 1986) (n = 24), which had involved participants with severe acne. The quality of the evidence was low due to very serious limitations of design in the study, more specifically unclear risk of selection, performance and detection bias and high risk of reporting bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
There were no available data for this outcome.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
One trial assessed this outcome and compared isotretinoin 1 mg/kg/d for 10 weeks followed by 0.5 mg/Kg/d for 10 weeks (n = 12) versus minocycline 100 mg/day followed by 50 mg/day for severe acne (n = 12) (Pigatto 1986). The proportion of participants who presented any less serious adverse effects was 67% higher in the isotretinoin group (RR 1.67, 95% CI 1.03 to 2.69; participants = 24; studies = 1). The quality of the evidence was very low due to very serious limitations of design (the study presented high risk of reporting bias and unclear risk of selection, performance, and detection bias) and imprecision of the effect (wide confidence interval).
Additionally, we presented the data on the frequency of each less serious adverse event reported by all included RCTs in Table 4; Table 5. For Pigatto 1986, data for each less serious adverse effect are in Table 4.
Secondary outcome: Dropout rates
There were no dropouts in the Pigatto 1986 study.
9. Oral isotretinoin versus oral tetracycline
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
The sole included RCT for this comparison, which enrolled people with severe acne, measured the improvement in acne severity assessed by a decrease in the number of cysts (Lester 1985). At the end of the therapy phase (16 weeks), participants on isotretinoin 1.0 to 2.0 mg/kg/day had a greater reduction in mean cysts count than those on tetracycline 0.5 g to 1 g/day but the confidence interval was wide and included 1 showing uncertainty (MD ‐2.80, 95% CI ‐7.90 to 2.30; participants = 30; studies = 1). After 24 weeks, on the follow‐up assessment, isotretinoin‐treated participants again had a greater decrease in the number of cysts than did the tetracycline‐treated group (MD ‐5.3, 95% CI ‐9.80 to ‐0.80; participants = 30; studies =1). The quality of evidence from both time points of measurement was very low due to very serious limitations in the study design (high risk of attrition bias and unclear risk of selection, performance and detection bias) and serious indirectness related to the outcome, as the trial measured the primary efficacy outcome of this review by assessing the clearance of cysts only, not of all inflammatory types of acne lesions (papules plus pustules, nodules, and cysts). Besides these reasons to downgrade the quality of both effects to very low, there was also serious imprecision (95% confidence intervals were wide).
Primary outcome: Frequency of serious adverse effects
Lester 1985 was the only included RCT in this comparison and did not detect any serious adverse event among participants with severe acne (n = 30). The quality of the evidence was low due to very serious limitations of design in Lester 1985, more specifically unclear risk of selection, performance, detection and other bias, and also high risk of attrition bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
There were no available data for this outcome.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
One trial assessed this outcome and compared oral isotretinoin 1.0 to 2.0 mg/Kg/day with tetracycline 0.5 g to 1 g/day for 16 weeks in participants with severe acne (Lester 1985). There was twice the risk of a less serious adverse effect among participants in the isotretinoin group (RR 2.07, 95% CI 1.22 to 3.51; participants = 30; studies = 1). The quality of evidence was downgraded to very low due to very serious limitations in the study design (high risk of attrition bias and unclear risk of selection, performance and detection bias) and serious imprecision (wide confidence interval). We entered data related to each less serious adverse event reported by the trial, in Table 4.
Secondary outcome: Dropout rate
Lester 1985 reported two dropouts due to poor control of the disease in the tetracycline group (2/15) and none in the isotretinoin group (0/15). Participants in the isotretinoin group had a lower risk of dropout but we are uncertain about this result because the confidence interval was wide and included 1 (RR 0.20, 95% CI 0.01 to 3.97; participants = 30; studies = 1) (Table 5). The quality of the evidence was very low due to very serious limitations of design in the trial (high risk of attrition bias and unclear risk of selection bias), besides serious imprecision of the effect (the wide confidence interval of the effect included the clinically important difference of 25%).
10. Oral isotretinoin versus oral dapsone
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
One included trial for this comparison assessed the outcome by measuring the reduction in lesion counts, but only in pustules, nodules, and cysts, not in all inflammatory lesion counts (Prendiville 1988). The RCT compared oral isotretinoin 40 mg/day versus oral dapsone 100 mg/day during 16 weeks for participants with severe acne. The report did not provide the exact values of the lesion counts at each time point of measurement. Authors just stated that participants on isotretinoin group had a lower number of lesions at 20 weeks, 28 weeks, and 36 weeks than the people in the dapsone group, with P values less than 0.05 at these three time points of measurement.
Primary outcome: Frequency of serious adverse effects
There were no serious adverse effects in participants of Prendiville 1988 (n = 40), which had addressed the comparison above in participants with severe acne. The quality of the evidence was low due to serious limitations of design in the trial, more specifically high risk of performance, attrition and reporting bias, and unclear risk of selection, detection and other bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
One RCT planned to assess this outcome but did not report the respective results of the comparison at any time point (Prendiville 1988).
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
There were no available data for this outcome.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
One trial assessed this outcome and compared oral isotretinoin 40 mg/day versus oral dapsone 100 mg/day for 16 weeks (Prendiville 1988) among participants with severe acne. The authors did not provide numeric data, but stated that all participants in the isotretinoin group presented at least one less serious mucocutaneous adverse event (cheilitis was the most notable). There was a hypersensitivity reaction (morbiliform eruption and abnormal liver function tests) in one participant in the dapsone group.
Secondary outcome: Dropout rates
Prendiville 1988 assessed this outcome, and the effect was equal for both interventions, oral isotretinoin 40 mg/day versus oral dapsone 100 mg/day, during 16 weeks, for participants with severe acne. Three participants on isotretinoin (3/20) and three in the dapsone group (3/20) dropped out of the study (RR 1.00, 95% CI 0.23 to 4.37; participants = 40; studies = 1). The quality of the evidence was downgraded by three levels due to very serious limitation of design in the trial (unclear risk of selection and detection bias and high risk of performance and attrition bias), besides serious imprecision (the effect had a wide confidence interval). Nonattendance or noncompliance were the reasons for dropouts in 1/20 in the dapsone group and 3/20 in the isotretinoin group. There was one dropout in the dapsone group due to dissatisfaction with treatment and another one due to hypersensitivity reaction. The effects related to the reasons for dropout are presented in Table 5.
11. Oral isotretinoin versus oral etretinate
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
Goldstein 1982 did not report any serious adverse event among all participants with severe acne (n = 56). The quality of the evidence was moderate due to serious limitations of design in the trial, more specifically unclear risk of selection, detection and attrition bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
There were no available data for this outcome.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
One trial assessed this outcome and compared isotretinoin 1 mg/kg/d for eight weeks (n = 28) versus etretinate 1 mg/kg/day for eight weeks in 28 participants with severe acne (Goldstein 1982). There was no difference between both interventions regarding the frequency of less serious adverse effects (RR 1.00, 95% CI 0.93 to 1.07; participants = 56; studies = 1). We considered the quality of evidence as being moderate due to serious limitations of design; the study had unclear bias for selection, detection, and attrition. We also presented data from each less serious adverse effect reported by the trial in Table 4.
Secondary outcome: Dropout rate
Goldstein 1982 reported six dropouts among 58 participants with severe acne. The loss to follow‐up occurred during the follow‐up period. However, the number of dropouts by intervention group was not reported.
12. Different doses/therapeutic regimens of oral isotretinoin
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (after at least 16 weeks)
Three studies assessed this outcome (Corlin 1984, Lee 2011, Strauss 1984). However, the data were heterogeneous (with multiple different comparisons of doses and therapeutic regimens in addition to different ways for the measurement of the outcome), and it was not possible to pool them in meta‐analysis.
In Corlin 1984, the decrease in total inflammatory lesion count (papules, pustules, and nodules) was 79% (mean number of lesions: 83 to 17), 80% (94 to 19) and 84% (105 to 17), respectively, for 0.05 mg/kg/daily (41 participants), 0.1 mg/kg/daily (54 participants) and 0.2 mg/kg/daily (59 participants) of oral isotretinoin after 20 weeks. Participants had severe acne. Neither standard deviation, P values or confidence intervals were available. The quality of the evidence was low due to very serious limitations of design in the RCT, more specifically high risk of performance and attrition bias and unclear risk of selection, detection, and other bias.
In Lee 2011, three doses were compared during 24 weeks in participants with moderate acne: (a) continuous (daily) low dose (0.25 to 0.4 mg/kg/day); (b) continuous conventional dose (0.5 to 0.7 mg/kg/day) and (c) intermittent regimen (i.e. there was no daily intake of the drug; the participant took the medicine only in some prespecified periods during the whole treatment) (0.5 to 0.7 mg/kg/day, first week in every four weeks). After 24 weeks, mean inflammatory lesion counts were lower in the continuous (daily) low dose regimen (MD 3.72 lesions, 95% CI 2.13 to 5.31; participants = 49; studies = 1) and continuous (daily) conventional dose (MD 3.87 lesions, 95% CI 2.31 to 5.43; participants = 49; studies = 1), when both were compared with the intermittent regimen. For both effects at 24 weeks, we downgraded the quality of evidence by two levels, from high to low, due to very serious limitations of design in Lee 2011 (unclear risk of bias regarding allocation concealment and high risk of performance and attrition bias). When comparing both continuous (daily intake) regimen groups, there was a greater effect with conventional dose regimen, but we are uncertain about this result because the confidence interval was wide and included 0 (MD 0.15 lesion, 95% CI ‐1.09 to 1.39; participants = 49; studies = 1). For this last measure at 24 weeks, the quality of the evidence was very low: besides the very serious limitations of design in Lee 2011, there was also the serious imprecision of the effect (the 95% confidence interval included the null effect and was wide).
In Strauss 1984, 150 participants with severe acne were included and the improvement in lesion count was defined as a 95% decrease in total inflammatory lesion count (greater than 4 mm) after 20 weeks. The compared doses were 0.1 mg/kg/d, 0.5 mg/kg/d and 1 mg/kg/d. Among participants of the group treated with the dose of 0.1 mg/kg/day, 58% achieved the 95% improvement in the total counting of facial and truncal inflammatory lesions (4 mm or greater in diameter), versus 80%, and 90% in both groups treated with higher doses (0.5 mg and 1 mg/kg/day, respectively). A reliable estimate of effect for the three different single pairwise comparisons was not possible due to lack of accurate denominators, as exact number of dropouts at the time point of this assessment was not clearly reported. The quality of the evidence was low due to very serious limitations of design in the study (high risk of attrition and reporting bias and unclear risk of selection, performance, and detection bias).
Primary outcome: Frequency of serious adverse effects
There was no report of any serious adverse event in 14 RCTs on different doses/regimens of oral isotretinoin (n= 906). The quality of the evidence was low due to very serious limitations of design in the studies (Agarwal 2011; Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; Dhaked 2016; Farrell 1980; Jones 1983a; Kapadia 2005; King 1982; Lee 2011; Shetti 2013; Strauss 1984; Van der Meeren 1983). These studies involved people with all severities of acne (mild, moderate, and severe), and the follow‐up of the outcome was the same as the duration of the therapy (ranged from 12 to 32 weeks, mean 20.9 weeks) plus the follow‐up after treatment ended (ranged from 0 to 48 weeks, mean 18.3 weeks).
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
There were no available data for this outcome.
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Five trials assessed this outcome (Ahmad 2015; Akman 2007; Dhaked 2016; Kapadia 2005; Lee 2011). However, the data were heterogeneous regarding the different doses and regimens of oral isotretinoin, and it was not possible to pool them in meta‐analyses.
Akman 2007 compared the effects of three different regimens of oral isotretinoin in participants with moderate and severe acne: (a) conventional dose of 0.5 mg/kg/d for 10 days of each month, for six months; (b) 0.5 mg/kg/d for one month, followed by 0.5 mg/kg/d for the first 10 days of each month for five months; (c) 0.5 mg/kg/d for six months. Authors used the FDA Global Grade, as described in (Cunliffe 2003), to assess acne improvement. They only reported a significant decrease of the acne grade in each treatment group at the end of the therapy phase (P < 0.001), with more higher grades of acne severity after cessation of oral isotretinoin in the group with the intermittent dose (conventional dose of 0.5 mg/kg/d for 10 days of each month, for six months) than in the group with conventional dose daily (0.5 mg/kg/d, for six months) at the end of the 12‐month follow‐up period (P = 0.002). The report did not provide the exact values of the acne grade in each intervention group for the measurements cited above.
Ahmad 2015 compared single (group 1) versus twice (group 2) daily doses of isotretinoin in participants with mild, moderate, and severe acne. The administered dose was from 0.5 to 1.0 mg/kg/d in both intervention groups during a mean period of 22 weeks. In the first month of therapy, participants received only half of the programmed maintenance dose to avoid exacerbation of the disease. The Global Acne Scoring (GAS) system was used to objectively classify acne cases in the study into mild (score 1 to 18), moderate (score 19 to 30), severe (score 31 to 38), and very severe (score > 39). The median GAS significantly decreased from 34 to 0 (P < 0.001) in group 1 and from 31 to 0 after treatment in group 2 (P < 0.001). Authors reported that there was no difference of effect between single and twice daily doses of isotretinoin at the end of therapy, with a P value of 0.8. More detailed numerical data for this effect were not provided.
Dhaked 2016 analysed isotretinoin 20 mg alternate days versus isotretinoin 20 mg/daily for 24 weeks in participants with moderate and severe acne. The lower dose (20 mg alternate days) improved acne severity 5% less than 20 mg/daily at the end of the therapy by the assessment of the number of participants who achieved an excellent treatment response (> 90% reduction in the lesion counts) (RR 0.95%; 95% CI 0.91 to 1.01; participants = 234; studies = 1); however, the confidence interval did include 1 showing uncertainty. We downgraded the quality of this evidence by two levels, from high to low, due to very serious limitations of design in the trial (unclear risk of selection, performance, and detection bias and high risk of attrition bias). At the 12‐week follow‐up period after the end of the therapy, the proportion of participants who maintained the excellent response to isotretinoin therapy was 18% lower in the 20 mg alternate days group (RR 0.82, 95% CI 0.72 to 0.92; participants = 234; studies = 1). The quality of the evidence was very low: there were very serious limitations of design in the RCT (as we have already cited above) and serious imprecision (the 95% confidence interval of the effect included the clinically important difference of 25% between interventions).
Kapadia 2005 compared isotretinoin 20 mg/day versus 40 mg/day for 24 weeks in participants with moderate to severe acne. The proportion of participants with complete clearing, excellent (> 80% clearing) or good (> 50% clearing) was 80% (24/30) with 20 mg/day and 86% (26/30) with 40 mg/day at all time points of measurement (8, 16 and 24 weeks). Participants receiving the higher daily dose of oral isotretinoin achieved a 8% greater improvement in acne severity than the group who received 20 mg/day in these assessments but the confidence interval included 1 showing uncertainty (RR 0.92, 95% CI 0.74 to 1.16; participants = 60; studies = 1). We considered the quality of the evidence as being low due to the very serious limitation of design in Kapadia 2005 (unclear risk of selection, performance, and detection bias; and high risk of reporting bias).
Lee 2011 studied three low dose isotretinoin regimens for moderate acne during 24 weeks: (a) continuous ‐ daily drug intake ‐ low dose (0.25 to 0.4 mg/kg/day); (b) continuous ‐ daily drug intake ‐ conventional dose (0.5 to 0.7 mg/kg/day); and (c) intermittent ‐ stopping and starting intake often over a month ‐ conventional dose (0.5 to 0.7 mg/kg/day for the first week in every four weeks). Authors used the Global Acne Grading System (GAGS), which classifies acne into mild (score 1 to 18), moderate (score 19 to 30), severe (score 31 to 38), and very severe (score > 39), to assess the improvement in acne severity according to physician's evaluation. The study provided mean values of the score in each intervention group at each time point of assessment of the outcome. Participants on therapy with the intermittent regimen had higher mean values of GAGS scores at the end of the treatment than participants on either continuous low dose (MD 1.57, 95% CI 0.12 to 3.02; participants = 60; studies = 1) or continuous conventional dose (MD 1.75, 95% CI 0.13 to 3.37; participants = 49; studies = 1). Treatment with continuous low doses of oral isotretinoin produced a higher mean final value of GAGS scores, i.e. it was less effective than therapy with continuous conventional doses, but the confidence interval did included zero showing some uncertainty (MD 0.18, 95% CI ‐1.44 to 1.80; participants = 49; studies = 1). The quality of the evidence from these three measures of effect was very low due to very serious limitations of design in the RCT (unclear risk of bias regarding allocation concealment and high risk of performance and attrition bias) and serious imprecision of the effect, which had a wide confidence interval. One year after the end of the therapy phase, participants on intermittent oral isotretinoin had higher mean values of GAGS scores than those participants who received either the continuous low dose regimen (MD 6.35, 95% CI 1.52 to 11.18; participants = 49; studies = 1) or the continuous conventional dose regimen (MD 7.93, 95% 3.33 to 12.53; participants = 49; studies = 1). Both analyses were of low‐quality due to the very serious limitations of design in the trial, which we have already described above. Participants who were in the continuous low dose intervention group presented higher mean values of GAGS scores than those ones in the continuous conventional dose group at the 12 months follow‐up measurement after the end of the treatment but the confidence interval included 0 showing uncertainty (MD 1.58, 95% CI ‐2.71 to 5.87; participants = 49; studies = 1). We downgraded the quality of this evidence to very low due to the very serious limitations of design in Lee 2011 and the imprecision related to the wide confidence interval of the effect.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Thirteen trials assessed this outcome (Agarwal 2011; Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; Dhaked 2016; Farrell 1980; Jones 1983a; Kapadia 2005; Lee 2011; Shetti 2013; Strauss 1984; Van der Meeren 1983). The data were heterogeneous regarding ways for the assessment of the outcome and also the many different doses and therapeutic regimens of oral isotretinoin. Therefore, we could not pool them in meta‐analyses. The numbers in detail for each less serious adverse event reported in each study are in Table 7. We have briefly described in each of the following paragraphs the results of this outcome by study, with the respective information about the severity of acne in the participants. The follow‐up of the outcome was the duration of the therapy (ranged from 12 to 32 weeks, mean 21.3 weeks) plus the follow‐up after treatment ended (ranged from 0 to 48 weeks, mean 19.8 weeks). It was not clear in any of the 13 studies how long treatment was given until the measure of the outcome, as all of them reported only the number of participants which developed each one of the less serious adverse effects during the entire follow‐up.
Agarwal 2011 compared four therapeutic regimens, of 16 weeks, for participants with mild, moderate, and severe acne: (a) continuous, 1 mg/kg/d; (b) alternate days, 1 mg/kg/d; (c) intermittent, 1 mg/kg/d for one week of each month; and (d) alternate days, 20 mg/d. Data related to the estimates of effect for the overall frequency of less serious adverse effects were not reported. The numbers for each less serious adverse event reported by the authors are presented in Table 7.
Ahmad 2015 compared single versus divided daily doses of oral isotretinoin (0.5 to 1.0 mg/kg/d) in participants with mild, moderate, and severe acne for a mean period of 22 weeks. During the initial month, only half of the anticipated maintenance dose was given to avoid acne exacerbation. The rate of less serious clinical adverse effects was higher (P = 0.04) with the single dose, including dry chapped lips, dry skin, eczema, epistaxis, dry eyes, gastrointestinal upset, angular stomatitis, back pain, ingrowing nail, cough, headache, pyogenic granuloma, and white hair. The report of the study did not provide more detailed information for this outcome.
Akman 2007 compared two different intermittent regimens with the traditional therapy regimen for moderate and severe acne: (a) conventional dose of 0.5 mg/kg/d for 10 days of each month, for six months; (b) 0.5 mg/kg/d for one month, followed by 0.5 mg/kg/d for the first 10 days of each month for five months; and (c) 0.5 mg/kg/d for six months. Authors reported that the frequency of each less serious clinical adverse event was higher in group (c). However, the report did not provide data related to the effect estimate of the overall frequency of less serious adverse effects. Regarding data of each less serious adverse event individually, we present the numbers in Table 7.
Corlin 1984 compared doses of 0.05 mg/kg/daily (64 participants), 0.1 mg/kg/daily (62 participants) and 0.2 mg/kg/daily (65 participants) oral isotretinoin, for 20 weeks, for severe acne. There was no report of data related to the estimate of the effect for the overall frequency of less serious adverse effects. We presented estimates of effect related to each less serious adverse event in Table 7.
Cumurcu 2009 compared conventional dose (> 0.5 mg/kg/day) with low dose (< 0.5 mg/kg/day) for 12 weeks. There was no report of data related to the overall frequency of less serious adverse effects. The numbers for each less serious adverse event, specifically ocular adverse effects, are presented in Table 7.
Dhaked 2016 analysed the comparison of isotretinoin 20 mg alternate days versus 20 mg/daily for moderate and severe acne for 24 weeks. There were no data related to overall frequency of less serious adverse effects; authors presented only estimates of effect related to each less serious adverse event. We presented these estimates of effect in Table 7.
Farrell 1980 compared doses of 0.1 mg/kg/d, 0.5 mg/kg/d and 1 mg/kg/d for 12 weeks, and the authors reported that there was no significant difference between treatment groups regarding incidence of clinical adverse effects. However, the report did not individualise the data of less serious adverse effects by intervention group. Also, there was no information regarding the overall frequency of less serious adverse effects for each intervention group.
Jones 1983a compared doses of 0.1 mg/kg/d, 0.5 mg/kg/d and 1.0 mg/kg/d for 16 weeks for moderate to severe acne, and there was no report of the overall incidence of less serious adverse effects in each group. We presented data of each less serious adverse event from this study in Table 7.
Kapadia 2005 compared doses of 20 mg/day and 40 mg/day for 24 weeks for moderate and severe acne, but the report of the study cited data regarding less serious adverse effects per intervention group only for mood changes. There was a higher incidence of mood changes in the group taking 40 mg/day. The numbers in detail for this specific less serious adverse event are presented in Table 7.
Lee 2011 compared three different therapeutic regimens for moderate acne, for 24 weeks: (a) continuous ‐ daily intake ‐ low dose (0.25 to 0.4 mg/kg/day); (b) continuous ‐ daily drug intake ‐ conventional dose (0.5 to 0.7 mg/kg/day); and (c) intermittent ‐ stopping and starting intake often over a month ‐ conventional dose (0.5 to 0.7 mg/kg/day for the first week in every four weeks). We made pairwise comparisons between the three treatment groups and participants receiving the intermittent conventional dose regimen had a 53% reduction in the risk of less serious adverse effects in the comparison with those ones on the continuous conventional dose group (RR 0.47; 95% CI 0.26 to 0.83; participants = 60; studies = 1). The risk of less serious adverse effects was 31% lower in continuous low dose group while compared to continuous conventional dose group (RR 0.69; 95% CI 0.48 to 1.00; participants = 60; studies = 1). In the comparison with participants on intermittent conventional dose, the group who received continuous low dose isotretinoin had a risk 32% higher for less serious adverse effects but we are uncertain about this result because the confidence interval included 1 (RR 0.68; 95% CI 0.35 to 1.30; participants = 60; studies = 1). The quality of the evidence for the last three effects was very low due to serious imprecision of the effects, which had wide confidence intervals, and very serious limitations of design in the study (unclear risk of bias for selection and high risk of bias for performance and attrition). In an additional analysis of each less serious adverse event, the risk of dry, chapped lips was 53% lower with intermittent conventional dose when compared with continuous conventional doses. More numbers in detail for each less serious adverse event are presented in Table 7.
Shetti 2013 compared low dose continuous oral isotretinoin with low dose intermittent oral isotretinoin for participants with moderate to severe acne. The study did not provide details of dose or duration of therapy. Also there was not an adequate report of numerical data related to less serious adverse effects.
Strauss 1984 compared doses of 0.1 mg/kg/d, 0.5 mg/kg/d and 1 mg/kg/d, for 20 weeks, for severe acne. There was no report of data related to the overall incidence of less serious adverse effects in each intervention group. We presented data from each less serious adverse event in Table 7.
Van der Meeren 1983 compared doses of 0.5 and 1.0 mg/kg/d for 12 weeks, for participants with severe acne. There was no difference between intervention groups regarding the overall frequency of less serious adverse effects (RR 1.00; 95 % CI 0.94 to 1.07; participants = 58; studies = 1). The quality of evidence was low due to very serious limitations of design in the study, which had unclear risk of bias for selection, performance, and detection and high risk of bias for attrition. Authors also reported that the average number of less serious adverse effects per participant was significantly higher (P = 0.002) with 1.0 mg/kg/d (6.4 events/participant versus 5.3 events/participant in the group with 0.5 mg/kg/d). The standard deviations for this assessment were not provided. Data from each less serious adverse event are presented in Table 7.
Secondary outcome: Dropout rates
Twelve trials assessed this outcome (Agarwal 2011; Ahmad 2015; Akman 2007; Corlin 1984; Cumurcu 2009; Dhaked 2016; Farrell 1980; Jones 1983a; Kapadia 2005; Lee 2011; Strauss 1984; Van der Meeren 1983). However, the data were heterogeneous (due to the large range of different doses and regimens which the studies had analysed) and it was not possible to pool them in meta‐analyses.
In Agarwal 2011, by analysis of the six pairwise comparisons between the four intervention groups, ((a) continuous, 1 mg/kg/d; (b) alternate days, 1 mg/kg/d; (c) intermittent, 1 mg/kg/d for one week of each month; and (d) alternate days, 20 mg/d), there was no difference between them regarding dropout rates. All six estimates of effect are represented in Table 6, with the groups which had lower dropout rates in bold when there was a difference in the effect. We considered all these analyses as being of very low‐quality due to the very serious limitations of design in Agarwal 2011 (high risk of performance, detection, and attrition bias) and serious imprecision of all six effects (wide confidence intervals). The study included participants with any degree of acne severity.
Ahmad 2015 and Kapadia 2005 reported no dropouts.
We made three pairwise comparisons involving the intervention groups of Akman 2007 ((a) conventional dose of 0.5 mg/kg/d for 10 days of each month, for six months; (b) 0.5 mg/kg/d for one month, followed by 0.5 mg/kg/d for the first 10 days of each month for five months; and (c) 0.5 mg/kg/d for six months). There was no difference between them regarding overall dropout rates. All three estimates of effect are presented in Table 6, and the groups which had lower dropout rates in each comparison are in bold when there was a difference in the effect. We considered the quality of the evidence from these three comparisons as being very low due to serious imprecision of the effects (wide confidence intervals) and very serious limitations of design in the study (unclear risk of bias for selection, performance, and detection, besides high risk of bias for attrition).
Corlin 1984 compared three daily doses (0.05 mg/kg, 0.1 mg/kg and 0.2 mg/kg) for 20 weeks in participants with severe acne. From analysis of the three possible pairwise comparisons between intervention groups, overall dropout rates were higher in the group on 0.05 mg/kg/daily when compared with both, 0.1 mg/kg/daily (RR 2.79; 95 % CI 1.35 to 5.75; participants = 191; studies = 1) and 0.2 mg/kg/daily (RR 3.89; 95 % CI 1.70 to 8.92; participants = 191; studies = 1). The quality of evidence for both effects was very low due to very serious limitations of design in the study (unclear risk of selection and detection bias and high risk of performance and attrition bias) and serious imprecision (both effects had a wide confidence interval). All three estimates of effect related to overall dropout rates, as other data regarding analysis of reasons for dropouts, are presented in Table 6.
There was no difference between participants receiving conventional daily dose (> 0.5 mg/kg) and those in the low daily dose group (< 0.5 mg/kg), both for 90 days, regarding overall dropout rates in Cumurcu 2009 (RR 1.04; 95% CI 0.07 to 15.74; participants = 26; studies = 1). The quality of the evidence was very low due to very serious limitations of design in the study (unclear risk of selection, performance, and detection bias and high risk of attrition and reporting bias) and serious imprecision of the effect (wide confidence intervals). Additional data from analysis of reasons for dropouts in this study are presented in Table 6.
In the study by Dhaked 2016, which compared isotretinoin 20 mg alternate days versus isotretinoin 20 mg/daily for moderate and severe acne for 24 weeks, there was twice the risk of dropout among participants on therapy with 20 mg alternate days; however, the confidence interval was wide and included 1 so we are uncertain about this result (RR 2.00, 95% CI 0.37 to 10.71; participants = 234; studies = 1). The quality of the evidence was very low due to the serious imprecision of the effect (wide confidence intervals) and the very serious limitations of design in the RCT, more specifically unclear risk of selection, performance, and detection bias and high risk of attrition bias.
Farrell 1980 reported two dropouts (2/14): one participant discontinued treatment due to hair loss and scalp desquamation (with no information about the intervention assigned) and one participant from isotretinoin 0.1 mg/kg/day (lowest dose) had a marked flare and was excluded.
Jones 1983a did not report detailed data related to dropouts from each one of the three intervention groups.
With data from the study by Lee 2011, which involved participants with moderate acne, we evaluated overall dropout rates by comparison of the three pairwise interventions: (a) continuous ‐ daily intake ‐ low dose (0.25 to 0.4 mg/kg/day); (b) continuous ‐ daily drug intake ‐ conventional dose (0.5 to 0.7 mg/kg/day); and (c) intermittent ‐ stopping and starting intake often over a month ‐ conventional dose (0.5 to 0.7 mg/kg/day for the first week in every four weeks). The quality of the evidence was very low due to serious imprecision of the three estimates of effect (which are presented in Table 6, with the groups which had lower dropout rates in bold when there was a difference in the effect) and very serious limitations of design in the study, which had unclear risk of bias for selection and high risk of bias for performance and attrition. Also in Table 6, we presented data for the reasons for dropout in Lee 2011.
From Strauss 1984, we analysed overall dropout rates between the three intervention groups (0.1 mg/kg/d, 0.5 mg/kg/d, and 1 mg/kg/d, for 20 weeks, for severe acne) by pairwise comparisons. The three estimates of effect are presented in Table 6, with the groups which had lower dropout rates in bold when there was difference in the effect. We considered the quality of the evidence as being very low, as all three estimates of effect had wide confidence intervals (serious imprecision) and the study had very serious limitations of design (unclear risk of selection, performance, and detection bias and high risk of attrition and reporting bias).
Van der Meeren 1983 involved participants with severe acne and compared doses of 0.5 and 1.0 mg/kg/d for 12 weeks. Overall dropout rates were higher among participants receiving 1.0 mg/kg/day group but we are uncertain about this result as the confidence interval included 1 (RR 1.16; 95% CI 0.28 to 4.73; participants = 58; studies = 1). The quality of the evidence was very low due to serious imprecision of the effect (wide confidence intervals) and very serious limitations of design in the study, which had unclear risk of bias for selection, performance, and detection and high risk of bias for attrition. Additional data related to reasons for dropouts from Van der Meeren 1983 are presented in Table 6.
13. Standard oral isotretinoin versus other formulations of oral isotretinoin
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (after at least 16 weeks)
Two trials assessed this outcome (Strauss 2001; Webster 2014). In both studies, authors stated that the other formulations of oral isotretinoin had as their main advantage the fact that they were not food‐dependent. The food‐dependency of absorption is an attribute of standard isotretinoin. Strauss 2001 compared standard isotretinoin 0.85 to 1.18 mg/kg/d with micronised isotretinoin 0.32 to 0.4 mg/kg/d (for 20 weeks, for participants with severe acne). The decrease in mean total inflammatory lesion count was greater with the standard formulation (MD ‐1.5 lesion, 95% CI ‐2.96 to ‐0.04; participants = 492; studies = 1). We downgraded the quality of this evidence from high to very low (by three levels) due to very serious limitations of design in the study (high risk of attrition and reporting bias) and imprecision of the effect (wide confidence intervals). Webster 2014 compared isotretinoin‐Lidose 1 mg/kg/d for 20 weeks with standard isotretinoin 0.5 mg/kg/d for the first 8 weeks, followed by 1 mg/kg/d until week 20, for participants with severe acne. There was no significant difference between the groups in terms of mean absolute decrease of facial and truncal nodule counts (16.4 with standard isotretinoin versus 16.8 with isotretinoin‐Lidose; 95% CI = ‐0,22 to 0.64). The authors reported that 81% (332/410) of participants receiving standard isotretinoin obtained a 90% improvement in nodule counts versus 76.9% (310/403) of participants in the isotretinoin‐Lidose group, but there was not a significant difference between the effects (RR 1.05, 95% CI 0.98 to 1.13; participants = 742; studies = 1). We considered this evidence as being of very low‐quality (downgrade of three levels) due to very serious limitations of design in Webster 2014 (high risk of attrition bias and unclear risk of selection bias) and serious indirectness, since the study analysed the primary efficacy outcome of this review by assessing a decrease in nodules only, not in all inflammatory types of acne lesions (papules plus pustules, nodules, and cysts).
Primary outcome: Frequency of serious adverse effects
Two studies assessed this outcome (Strauss 2001; Webster 2014). Strauss 2001 (n = 602) reported six serious adverse effects; however, they were considered as "remotely related" (two cases of appendicitis with standard isotretinoin) or unrelated to isotretinoin use (one accident and one worsened diabetes with standard isotretinoin, and one terminated pregnancy and one accident with micronised isotretinoin). The quality of the evidence was low due to very serious limitations of design in Strauss 2001, more specifically high risk of attrition and reporting bias and unclear risk of selection, performance, detection, and other bias. Webster 2014 (n = 925) reported one serious psychiatric adverse event (substance abuse) with isotretinoin‐Lidose. The quality of this last analysis was low due to very serious limitations of design in the trial, more specifically high risk of attrition bias and unclear risk of selection bias.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
One trial assessed this outcome (Strauss 2001). Strauss 2001 found no difference in effects between both groups (after 20 weeks) in the proportion of participants with complete clearing or excellent improvement (almost clear) in acne severity: 91% (221/241) with standard isotretinoin and 90% (228/251) with micronised isotretinoin (RR 1.01, 95% CI 0.96 to 1.07; participants = 492; studies = 1) at 12 to 20 weeks after treatment. We downgraded the quality of this analysis by two levels, from high to low, due to the very serious limitations of design in the study (high risk of attrition and reporting bias).
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
Two trials assessed this outcome in participants with severe acne who received isotretinoin during 20 weeks (Strauss 2001; Webster 2014). There was no difference between the effect of standard isotretinoin and the other formulations, with high rates of participants with complete clearing or excellent improvement (almost clear) in acne severity after the therapy in both studies (RR 1.06, 95% CI 1.00 to 1.11; participants = 1274; studies = 2; I2= 0%) (Analysis 3.1). The quality of this evidence was downgraded two levels, from high to low, due to the very serious limitations of design in both studies (high risk of attrition and reporting bias in Strauss 2001, and high risk of attrition bias in Webster 2014).
3.1. Analysis.
Comparison 3 Standard oral isotretinoin versus other formulations of oral isotretinoin, Outcome 1 Improvement in acne severity assessed by physician's global evaluation.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
There were no available data for this outcome.
Secondary outcome: Frequency of less serious adverse effects
Two studies assessed this outcome (Strauss 2001; Webster 2014). In Strauss 2001, the overall frequency of less serious adverse effects during a 20‐week therapy phase was similar for both formulations (micronised and standard isotretinoin) in participants with severe acne (RR 1.01; 95% CI 0.99 to 1.03; participants = 600; studies =1). The quality of the evidence was low due to very serious limitations of design in the study (unclear risk of selection, performance, and detection bias, besides high risk of attrition and reporting bias). Webster 2014 reported a 4% lower risk of less serious adverse effects with standard isotretinoin in the comparison with isotretinoin‐Lidose, for 20 weeks, for severe acne but the confidence interval included 1 (RR 0.96; 95% CI 0.91 to 1.02; participants = 925; studies = 1). The quality of evidence was low due to very serious limitations of design in the study (unclear risk of selection bias and high risk of attrition bias). We did not pool data from these studies due to substantial statistical heterogeneity (I2 = 52%). Data related to each clinical or laboratory related less serious adverse event from both studies are in Table 4.
Secondary outcome: Dropout rates
Two studies, which compared different formulations of oral isotretinoin against the standard formulation during a 20‐week therapy period, assessed the outcome (Strauss 2001; Webster 2014). Results from both were pooled in meta‐analysis. The overall dropout rates were equal for standard isotretinoin and other formulations (RR 1.00, 95% CI 0.74 to 1.34; participants = 1527; studies = 2; I2 = 37%) (Analysis 3.2), but there was heterogeneity. By analysing results from each study separately, there was 16% greater overall dropout rates with micronised isotretinoin than with standard isotretinoin but the confidence interval included 1 showing uncertainty (RR 1.16, 95% CI 0.83 to 1.63; participants = 600; studies = 1) (Strauss 2001); and a 14% lower risk of dropout with standard isotretinoin in the comparison with isotretinoin‐Lidose, again with the confidence interval including 1 showing uncertainty (RR 0.86, 95% CI 0.63 to 1.19; participants = 925; studies = 1) (Webster 2014). Dropout rates due to adverse effects were 11% lower with standard isotretinoin than with other formulations but we are uncertain about this result as the confidence interval included 1 (RR 0.89, 95 % CI 0.56 to 1.43; participants = 1527; studies = 2; I2 = 0% ) (Analysis 3.2). Together, rates of dropouts due to loss to follow‐up, non‐compliance, or withdrawal of consent were 10% higher among participants on standard isotretinoin but since the confidence interval includes 1 we are uncertain of this result (RR 1.10, 95% CI 0.82 to 1.48; participants = 1527; studies = 2; I2 = 0%) (Analysis 3.2). We downgraded the quality of evidence for all these five effects cited above from high to very low (by three levels) due to the very serious limitations of design in both studies (high risk of attrition and reporting bias in Strauss 2001, and high risk of attrition bias in Webster 2014) and the serious imprecision of the effects, which presented wide confidence intervals. Numbers in detail for dropouts are presented in Table 5.
3.2. Analysis.
Comparison 3 Standard oral isotretinoin versus other formulations of oral isotretinoin, Outcome 2 Dropout rates.
14. Oral isotretinoin versus placebo
Primary outcome: Improvement in acne severity assessed by a decrease in total inflammatory lesion count (treatment for at least 16 weeks)
There were no available data for this outcome.
Primary outcome: Frequency of serious adverse effects
No serious adverse effects were detected in participants from both included RCTs which compared oral isotretinoin with placebo: Peck 1982 (n = 33) and Rademaker 2013b (n = 60). The quality of the evidence was low due to very serious limitations of design in both trials: high risk of reporting bias, and also unclear risk of selection, performance, detection, and other bias in Peck 1982; high risk of attrition bias and unclear risk of selection and other bias in Rademaker 2013b.
Secondary outcome: Improvement in acne severity assessed by participant's self‐assessment of acne severity
One trial assessed this outcome (Rademaker 2013b). The study only reported that there was a statistical difference in favour of the isotretinoin group after 16 weeks (P = 0.03).
Secondary outcome: Improvement in acne severity assessed by physician's global evaluation
There were no available data for this outcome.
Secondary outcome: Changes in quality of life (QoL) assessed using a validated instrument
One trial assessed this outcome and compared oral isotretinoin 5 mg/day with placebo for 16 weeks in participants with low‐grade acne (Rademaker 2013b). Investigators used the Dermatology Life Quality Index (DLQI), a validated questionnaire in which higher scores meant a worse quality of life, to assess the outcome. There was a greater decrease in mean DLQI score with isotretinoin after 16 weeks of therapy (MD ‐2.70, 95% CI ‐4.13 to ‐1.27; participants = 58; studies = 1). We downgraded the quality of this evidence from high to very low (three levels) due to very serious limitations of design in the study (high risk of attrition bias and unclear risk of selection bias) and serious imprecision of the effect (wide confidence intervals).
Secondary outcome: Frequency of less serious adverse effects
Two trials assessed this outcome (Peck 1982; Rademaker 2013b). Peck 1982 compared oral isotretinoin at a minimum of 0.5 mg/kg/d with placebo. At the monthly visit, participants who did not improve or had a mild worsening of acne had their doses of isotretinoin or placebo increased by 0.5 mg/kg/day. When there was intense worsening of acne in participants on placebo at this same time point of measurement, the investigators followed the study protocol and moved the participants to the isotretinoin group. There were no numerical details of the frequency of each adverse event observed at the first double‐blind phase. Authors only reported the occurrence of mucocutaneous alterations, arthralgia, decreased appetite, fatigue, sunburn, raised liver function tests, and hypertriglyceridaemia among participants who received isotretinoin.
In Rademaker 2013b, there was a 4.31 times higher risk of a less serious adverse event among participants with low‐grade acne receiving oral isotretinoin in comparison with participants in the placebo group after 16 weeks but the confidence interval was very wide and included 1 showing great uncertainty (RR 4.31; 95% CI 0.45 to 41.09; participants = 60; studies = 1). The quality of evidence was very low due to very serious limitations of design in the study (high risk of attrition bias and unclear risk of selection bias) and serious imprecision of the effect (wide confidence interval). The numbers in detail for each less serious adverse event are presented in Table 4.
Secondary outcome: Dropout rates
Two studies assessed this outcome (Peck 1982; Rademaker 2013b). Peck 1982 reported no dropouts. Rademaker 2013b reported 14 dropouts in overall and did not specify the number of dropouts by group. The reasons were loss to follow‐up (n = 5), adverse effects (n = 2), not specified (n = 1), and temporary exclusion due to minor protocol violations (n = 8).
Discussion
Summary of main results
This systematic review provided a comprehensive analysis of the best available evidence on efficacy and safety of oral isotretinoin for acne. We addressed concerns regarding external and internal validity of the 31 included RCTs in 3836 people. We considered oral isotretinoin versus oral antibiotics plus topical agents as the main comparison in this review.
Study participants ranged in age from 12 years to 55 years and in acne severity from mild to severe, although most participants had severe acne. There were twice as many male than female participants. Study duration was not clearly reported in most studies; based on those that did, the mean duration was 23 months.
Most of the included studies compared different doses or regimens of oral isotretinoin (a further two studies assessed standard oral isotretinoin against different formulations of oral isotretinoin), and about a quarter compared isotretinoin against antibiotics (with or without topical treatments). Two studies compared isotretinoin against placebo, one against a retinoid, and two assessed oral isotretinoin with versus without topical agents.
For our main comparison, there were only three RCTs (Gollnick 2001; Oprica 2007; Tan 2014), which involved 400 people with moderate and severe acne. None of the three trials had reported data specifically related to severe recalcitrant acne. From meta‐analysis of the data from these three studies, we found the following very low‐quality evidence related to the assessment of our primary outcomes, which had a very short‐term follow‐up (20 to 24 weeks of therapy) (outcome assessment was immediately at the end of treatment):
there was no difference between oral isotretinoin and oral antibiotics plus topical treatments regarding efficacy in clearing acne inflammatory lesions during therapy phase; and
isotretinoin led to one participant developing Stevens‐Johnson syndrome, whereas no participants experienced serious adverse effects in the oral antibiotics plus topical treatment group (Table 1).
However, we are uncertain of these conclusions due to the very low‐quality of this evidence, so these results neither support nor refute the common recommendation that oral isotretinoin is the most effective available acne treatment if administered for a mean period of 20 weeks. Our findings did not directly challenge recent acne therapy guidelines, where isotretinoin is a first‐line medication for moderate to severe acne that does not respond to systemic antibiotic therapy plus topical therapy (Gollnick 2016; Nast 2010; Thiboutot 2009; Zaenglein 2016).
Findings related to two secondary efficacy outcomes of our review (improvement of acne at the end of the treatment measured by physician global evaluation of acne severity and frequency of less serious adverse effects) showed there may be higher efficacy of oral isotretinoin in the comparison with antibiotics plus topical agents, but more adverse effects of a less serious nature, such as severe or persistent skin symptoms, or both (e.g. dry lips, dry skin, and cheilitis), and systemic events (e.g. vomiting and nausea) (low‐quality evidence).
Due to lack of data from RCTs in the current literature, our review could not provide evidence related to either of our primary efficacy outcomes, or any other of our outcomes, in long‐term follow‐up.
Also, as none of the three included RCTs was restricted to participants with severe recalcitrant acne, uncertainties are still present regarding oral isotretinoin in improving acne severity and quality of life during the therapy phase and, especially, in long‐term follow‐up for this subset of acne participants.
When different doses/regimens of oral isotretinoin were assessed, three studies comparing different doses of isotretinoin measured the primary outcome ‘Improvement in acne severity assessed by a decrease in total inflammatory lesion count’; this was done immediately after 20 to 24 weeks of therapy.
Two of these studies (304 participants with severe acne) compared three different doses and observed greater improvement with the higher dose (low‐quality evidence). The third study (60 participants with moderate acne) compared three different doses and therapeutic regimens and showed that continuous (daily) low dose and continuous (daily) conventional dose improved acne more (low‐quality evidence for both effects) than the intermittent regimen. Conventional dose reduced inflammatory lesion counts more than low dose; however, we are uncertain of this result because it was based on very low‐quality evidence.
In the trials of different doses/regimens of oral isotretinoin, participants had moderate to (mainly) severe acne, although a small number had mild acne. There were no serious adverse effects in 14 trials (906 participants) that assessed safety outcomes (low‐quality evidence); treatment duration lasted 12 to 32 weeks, with follow‐up up to 48 weeks after treatment stopped. Severity of acne measured by a doctor was not assessed in this comparison. Thirteen trials (858 participants) analysed frequency of less serious adverse effects, such as skin dryness, hair loss, and pruritus. However, assessment of this outcome was very heterogeneous among the studies and this precluded evidence synthesis; this means we cannot draw certain conclusions (low‐ to very low‐quality evidence, where assessed).
None of the 31 included RCTs reported birth defects but potential teratogenic effects are recognised as a limitation to use of oral isotretinoin.
Serious adverse effects reported by nonRCTs
Additionally, we made a qualitative synthesis from nonRCTs (cohort and case‐control) with a focus on serious adverse effects, to assess serious adverse effects and controversies regarding isotretinoin safety, especially psychiatric outcomes and inflammatory bowel disease. Due to the low frequency of these outcomes, the inclusion of observational studies might constitute the best approach to explore them. These two serious safety outcomes have been the cause of concern among people with acne, clinicians, and decision‐makers around the world. Numerous personal injury lawsuits, usually involving high costs and especially in the United States, have already claimed the association of either psychiatric or inflammatory bowel disease with oral isotretinoin use (Williams 2012).
Psychiatric disease
Among the uncertainties about the use of oral isotretinoin for acne, the UK DUETs highlights the possible association between oral isotretinoin and depression and suicidal behaviour in acne patients (DUETs 2010). This review found eight nonrandomised comparative studies (Azoulay 2008; Chia 2005; Cohen 2007; Jick 2000; Kaymak 2009; McGrath 2010; Ng 2002; Sundstrom 2010) that evaluated psychiatric safety issues in participants with acne who had used oral isotretinoin. Table 3 presents data from these nonRCT studies and also a qualitative synthesis we conducted by applying an extension for nonrandomised studies to the Cochrane's 'Risk of bias' tool, according to the report by Turner 2013. Interestingly, in one of eight nonRCTs included in this review, there was a significant reduction of the level of depression within the oral isotretinoin group when compared with those on topical therapy (Kaymak 2009). One retrospective case‐control study found that exposure to isotretinoin within five months immediately prior to the first diagnosis or hospitalisation for depression was significantly higher than in a five‐month control period (Azoulay 2008). However, most of the nonRCTs did not find a significant difference in the frequency of depression between oral isotretinoin and other active oral or topical treatment (Chia 2005; Cohen 2007; Jick 2000; McGrath 2010; Ng 2002; Sundstrom 2010). Among these eight nonRCTs, two assessed frequency of suicide and did not find a significant difference between the groups (Jick 2000; Sundstrom 2010). All the nonRCTs, the cohorts and case‐control studies assessing depressive outcomes, were at high risk of bias for at least one criteria of the internal validity assessment. This fact reduced the quality rating of this body of evidence to very low, as nonRCTs start as low‐quality evidence according to the approach of The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group (Guyatt 2011).
Inflammatory bowel disease
There was no case of Inflammatory bowel (IBD) disease reported in the 26 RCTs included in this review. From the additional search for nonrandomised studies on adverse effects, and also from references from our searching for RCTs, we found six nonrandomised controlled studies on oral isotretinoin and the risk of inflammatory bowel disease (Alhusayen 2013; Bernstein 2009; Crockett 2010; Etminan 2013; Racine 2014; Rashtak 2014). Among these studies, Crockett 2010 found a significant association between oral isotretinoin and ulcerative colitis (but not Crohn's disease), and Alhusayen 2013 detected an association between oral isotretinoin and IBD in participants aged 12 to 19 years only after a prespecified subgroup analysis by age. However, in all other four studies (Bernstein 2009; Etminan 2013, Racine 2014; Rashtak 2014), there was no increased occurrence of IBD associated with the use of oral isotretinoin. Instead, in Rashtak 2014, there was a decreased risk of IBD with isotretinoin exposure; and in Racine 2014, isotretinoin use was associated with a lower risk of ulcerative colitis. We did not assess risk of bias, nor the quality of evidence for these studies on oral isotretinoin and IBD.
We did not find reports of any other serious adverse effect among nonrandomised studies analysed within the additional screening process.
Overall completeness and applicability of evidence
Our review has limitations in terms of its external validity, and these relate to the following:
scarcity of data related to some types of participants, such as very young adolescents (our results related to people from 12 to 55 years) and people with truncal or recalcitrant acne;
few studies addressing both our primary efficacy outcomes, "improvement in acne severity assessed by a decrease in total inflammatory lesion count", and participant‐reported outcomes, such as "improvement in acne severity" assessed by the participant’s self‐assessment, as well as "changes in quality of life";
very few studies focused on our main comparison, oral antibiotics plus topical agents versus oral isotretinoin, and also there was low‐quality data from studies on the comparison of different doses or regimens of oral isotretinoin; and
most of the evidence was supported by data from short‐term follow‐up.
The included studies overall had a very short follow‐up period (the mean being 26.5 weeks after the end of the therapy phase), precluding a long‐term evaluation of efficacy outcomes.
Approximately a third of included studies addressed our primary outcome "improvement in acne severity". The secondary efficacy outcome, "improvement in acne severity", evaluated by participant self‐assessment, was measured in only four included RCTs, and only two studies assessed changes in quality of life using a validated instrument.
Daily low dose and intermittent regimens of therapy with oral isotretinoin, which might be safer for patients, have been frequently adopted to treat not only severe, but also moderate and mild acne (Zaenglein 2016). We found 14 RCTs that compared different doses or regimens of oral isotretinoin. Once again, it was not possible to pool the data and produce more robust evidence to answer this question. There was either insufficient reporting of data or great heterogeneity regarding doses and regimens, to an extent that grouping their data would not produce clinically sensible results. Only one from these 14 studies provided data on our primary efficacy outcome in an adequate way to make possible a measurement of the effect (Lee 2011), and also only one of these 14 RCTs included participants with mild acne (Agarwal 2011).
According to the UK DUETs (DUETs 2009), one of the concerns raised by professionals dealing with acne is the true benefit of early intervention with oral isotretinoin versus alternative treatments for moderate or mild acne. As a result of the lack of evidence derived from RCTs, this review could not provide an answer to this question either.
Regarding the frequency of less serious adverse effects, there was considerable heterogeneity in the means of monitoring, assessing, and measuring the clinical and laboratory adverse effects among included RCTs. This fact precluded pooling of data from specific types of less serious adverse effects, such as the mucocutaneous alterations or arthralgia, for example. The available data could not provide any evidence related to long‐term adverse effects that were possibly related to oral isotretinoin, another concern that is very often raised among acne patients (DUETs 2007). Although all but two of the included studies assessed the frequency of serious (our primary safety outcome) or less serious (our secondary safety outcome) adverse effects in the short‐term, and all but three of the studies addressed dropout rates, the quality of the evidence meant we could not draw firm conclusions.
Furthermore, we could not provide any evidence on the effect of oral isotretinoin in specific subgroups, such as very young adolescents or people with truncal acne or more severe recalcitrant disease (including severe recalcitrant nodular acne), i.e. those that represented subgroups that seem to have the poorest response to the drug and are more likely to relapse (Nast 2010). Also, all included RCTs were carried out in dermatology clinics, which could limit the extension of findings to different settings, such as those visiting general clinicians.
According to the UK Database of Uncertainties about the Effects of Treatments (DUETs), one of the gaps in scientific knowledge about oral isotretinoin for acne is the comparison between the use of low dose rather than standard dose (DUETs 2011). Also, alternatives to the daily prescription of oral isotretinoin for acne have become more frequent in clinical practice due to the serious safety concerns related to the drug which have been specially discussed in the last decade in both the general and scientific media. In this review, we analysed different doses/therapeutic regimens of oral isotretinoin. Two studies (both from the 1980s, out of 12 RCTs that were from the 80s; these older studies evaluated and presented efficacy and safety outcomes in a very heterogeneous way) compared different daily doses during 20 weeks for participants with severe acne. However, these studies could not provide a reliable measurement of the effect due to both large attrition bias and lack of numerical data (Corlin 1984; Strauss 1984). In a more recent trial involving 60 people, Lee 2011 compared three different therapeutic regimens for 24 weeks (continuous low dose (0.25 to 0.4 mg/kg/day), continuous conventional dose (0.5 to 0.7 mg/kg/day) and intermittent dose (0.5 to 0.7 mg/kg/day, first week in every 4 weeks)); the study found low‐ and very low‐quality evidence, respectively, of: 1) higher decreases in inflammatory lesion counts after 24 weeks with both continuous regimens (conventional and low dose) when each one of them were compared with the intermittent regimen; and 2) greater effect of continuous conventional dose than continuous low dose in reducing total inflammatory lesion count at 24 weeks of therapy.
Quality of the evidence
For our seven different primary and secondary outcomes, the quality of evidence ranged from moderate to very low. A downgrade of at least one level, from high to moderate quality according to GRADE, was present in all effects we analysed due to serious limitations of design in the included studies. This review included 31 RCTs, which involved a total of 3836 participants, and all these trials presented unclear or high risk of bias for at least two of the seven domains of risk of bias analysed by Cochrane's 'Risk of bias' tool (Figure 3). Over half the trials were at high risk of attrition bias, and presented an important potential impact of missing data on effect estimates. Additionaly, for most outcomes, we downgraded by one more level the quality of the evidence, from low to very low, due to serious imprecision of the effects caused by the wide confidence intervals which included the clinically relevant difference of 25% between interventions as well as no clinically relevant difference between the interventions. Due to indirectness, we downgraded by one level the evidence related to our primary efficacy outcome and the main comparison of the review, oral isotretinoin versus oral antibiotic plus topical agents: in two of the three included RCTs, the outcome was assessed by counting only one or two types of inflammatory acne lesions, not all of them (Table 1). It was not possible to address publication bias in our review due to the low number of studies contributing to each of our estimates of effect; no measurement in this review has included data from more than three RCTs. The very low‐quality body of evidence identified by this review did not allow a robust and definitive conclusion about the true efficacy and safety of oral isotretinoin, i.e. we still have uncertainty about all the effects of oral isotretinoin for acne.
Potential biases in the review process
Potential bias might emerge from any differences between our previous published protocol (where we prespecified the research question and inclusion criteria for studies) and this review, but we clearly described these minor deviations from our protocol in the Differences between protocol and review section. The application of the GRADE system (Guyatt 2011) to evaluate the quality of evidence related to each effect measured by our review was not prespecified in our protocol. In fact, Cochrane methodology did not incorporate GRADE assessments until after our protocol publication. To avoid any potential bias related to study selection, we had all articles written in other languages translated to English during the screening process, and before the decision regarding eligibility. Two of us independently screened references and assessed the studies for eligibility, which also occurred in the processes of data extraction and evaluation of risk of bias of the included studies. A third opinion from a different author resolved disagreements during the study selection, data extraction, and 'Risk of bias' assessment. Despite not having designed a funnel plot to assess the likelihood of publication bias of the included studies, we adopted other procedures to avoid any potential bias in our review process due to this issue, such as performing a comprehensive search for ongoing studies and handsearching conference proceedings. We also made efforts to obtain unpublished protocols and additional data from all of our included RCTs, especially from those ones for which we detected a high risk of reporting bias. Potential bias could rise from our results and assessments of quality related to included RCTs that had poor reporting of data. When relevant data were missing or poorly presented, we clearly described this within our review sections. Also, the absence of planned subgroup analyses to investigate heterogeneity in our review could be seen as a source of bias. However, as we have explained in the Differences between protocol and review section, the scarcity of data from included studies did not allow us to perform subgroup analyses regarding severity of acne, treatment duration, level of improvement in acne severity, age, and gender.
Agreements and disagreements with other studies or reviews
We identified only one non‐Cochrane systematic review (Lehmann 2001), which had assessed the efficacy and safety of oral isotretinoin for acne in clinical trials. In Lehmann 2001, authors stated that methodological limitations, multiple treatments, and heterogeneity regarding assessment of outcomes precluded quantitative data synthesis from the trials on oral isotretinoin versus other anti‐acne therapies, placebo, or itself in different doses, and this fact limited their conclusions. We have also found the same limitations in our analysis of our included RCTs that were also analysed by Lehmann. The review by Lehmann 2001, however, presented only a narrative summary of the results from trials and did not provide estimates of effect measures related to outcomes from the trials. None of the trials included in the review by Lehmann 2001 reported birth defects, and this finding is consistent with our findings. The review, a publication on the management of acne from a governmental agency, was available for historical reference only, since it was never updated. Lehmann 2001 was systematically reviewed, but lacked risk of bias assessment for each included study and clear criteria for quality of evidence assessment. Also, there were no prespecified outcomes; the searches for clinical trials did not address studies in languages other than English, and nonrandomised controlled clinical trials were included.
A Cochrane review, Garner 2012, assessed the efficacy and safety of oral isotretinoin in comparison with oral minocycline for acne and, consistent with our review, found that isotretinoin was equivalent to minocycline plus topical azelaic acid in reducing acne inflammatory lesion counts after 24 weeks of treatment. Garner 2012 also found isotretinoin was associated with less serious adverse effects than minocycline after four weeks of therapy. We also found four guidelines on acne management published in the last ten years (Gollnick 2016; Nast 2010; Thiboutot 2009; Zaenglein 2016). These studies did not perform a systematic and thorough extraction of data, nor assessment of quality of the evidence which supported their recommendations, as we have done in applying the GRADE guidelines (Guyatt 2011) in this review.
Four Cochrane reviews on interventions for acne (Arowojolu 2012; Barbaric 2016; Cao 2015; Garner 2012) commented on the considerable heterogeneity in measurement of efficacy outcomes related to acne among the available randomised trials, consistent with our findings. These reviews detected few studies which evaluated participant‐reported outcomes and quality of life, a fact which we have also noted.
We retrieved three systematic reviews of the literature (Kontaxakis 2009; Magin 2005; Marqueling 2005) which addressed the concern of the association between oral isotretinoin for acne and psychiatric symptoms. The three studies concluded that, based on the best available evidence, it was not possible to establish a definitive causal relationship. This is consistent with the findings of our additional search for serious adverse effects in nonRCTs studies (nonrandomised clinical trials, cohorts, and case‐control), but we are uncertain of the validity of this data as it is very low‐quality evidence. However, none of these reviews reported prespecified outcomes, nor performed quality assessments of the evidence according to clear and well‐defined criteria. In Magin 2005, the search was restricted to studies in English. There was no clear description of the study selection process in the reviews by Kontaxakis 2009 and Magin 2005. We found two meta‐analyses of population studies on oral isotretinoin and inflammatory bowel disease (IBD) (Etminan 2013; Lee 2016). Both had addressed the same six studies we retrieved in our review and concluded that there was no evidence of any association between oral isotretinoin and occurrence of IBD. Lee 2016 had restricted searching and selection to articles in English. In Etminan 2013, the searches for studies in databases were restricted to the period from 2000 through May 2012. Both studies (Etminan 2013; Lee 2016) did not report prespecified outcomes.
Despite consensus recommendation for avoidance of the use of topical anti‐acne treatment together with isotretinoin (Gollnick 2016), as this may potentiate the occurrence of mucocutaneous side effects of oral isotretinoin, two of our included RCTs analysed benefits and harms of oral isotretinoin together with anti‐acne topical agents to treat moderate and severe acne (Dhir 2008; Faghihi 2014). Neither of these two studies assessed our primary efficacy outcome. There was no serious adverse event in participants from either trial (low‐quality evidence).
Systemic antibiotics as monotherapy are not recommended and also do not seem to be a usual option in clinical practice (Gollnick 2016; Nast 2010; Thiboutot 2009; Zaenglein 2016). However, five included trials of this review, with 244 people, compared oral isotretinoin to tetracycline (Lester 1985), minocycline (Pigatto 1986), dapsone (Prendiville 1988), azithromycin (Wahab 2008), and erythromycin (Jones 1983b); four of them did not provide data for our primary efficacy outcome. From one RCT, which involved 30 participants (Lester 1985), we found very low‐quality evidence that isotretinoin produced a greater improvement in acne severity than tetracycline on follow‐up assessment at 16 and 24 weeks, after a 16 weeks of therapy. Regarding harms, there were no serious adverse effects among participants on either oral isotretinoin or oral antibiotics in these five trials (low‐quality evidence).
The classical indication for the use of oral isotretinoin since its launch on the market remains in moderate to severe acne that does not respond to the combination of oral antibiotic plus topical agents. The superiority of oral isotretinoin in achieving a prolonged remission, and even a cure, of more severe cases is a well‐accepted concept among dermatologists around the world (Gollnick 2016; Nast 2010; Thiboutot 2009; Zaenglein 2016). Due to design limitations in the three RCTs that investigated the main comparison of our review (Gollnick 2001; Oprica 2007; Tan 2014), our findings demonstrate a lack of high‐quality RCT support for this view in acne management. Our findings do not challenge this view either, and our findings do not challenge current treatment approaches in acne.
Authors' conclusions
Implications for practice.
The current recommendation of clinical guidelines that oral isotretinoin should be the first‐line treatment for moderate to severe acne unresponsive to previous therapies, being more effective than the use of oral antibiotics plus topical agents, underpins current dermatological practice. The findings of this review do not challenge that recommendation. However, the low‐ or very low‐quality evidence means that the clinical trials in this area are not able to more precisely define the role of isotretinoin in practice. Unfortunately, due to the scarcity of data, and mainly short‐term follow‐up of the randomised controlled trials (RCTs), this review does not provide a definitive evidence‐based conclusion about the efficacy and safety of oral isotretinoin in the two most clinically important comparisons for acne treatment (oral isotretinoin compared with oral antibiotics plus topical agents for acne, and different doses/regimens of oral isotretinoin).
Regarding the best dose regimen, together with daily or intermittent use of oral isotretinoin, the three included studies were too heterogeneous with respect to isotretinoin doses.
Due to very low‐quality evidence, RCTs do not provide certainty about how much oral isotretinoin improves acne severity assessed by a decrease in total inflammatory lesion count or whether isotretinoin increases the frequency of serious adverse effects when compared with oral antibiotics plus topical agents for acne. Based on low‐quality evidence, isotretinoin may slightly improve acne severity assessed by physician's global evaluation, but it may lead to more less‐serious adverse effects, such as local events (severe or persistent skin symptoms, or both, such as dry lips, dry skin, and cheilitis) and systemic events (vomiting and nausea).
When using different doses/regimens of oral isotretinoin, there was low‐quality evidence that a higher continuous dose may improve acne severity when compared to a lower or intermittent dose, with no serious adverse effects found. Severity of acne measured by a doctor was not assessed in this comparison. It was not possible to synthesise evidence regarding the frequency of less serious adverse effects due to heterogeneity in the assessment of this outcome, so we are uncertain if there are differences between the dosing groups. Events reported included skin dryness, hair loss, and pruritus (low‐ to very low‐quality evidence, where assessed).
We have scarce RCT evidence about the effects of oral isotretinoin against the following: placebo, oral etretinate, and oral isotretinoin associated with topical agents.
The very low‐quality of the evidence (from all RCTs and nonRCTs) did not allow a definitive conclusion about a higher risk of psychiatric outcomes, including suicide attempts and inflammatory bowel disease associated with oral isotretinoin use.
Implications for research.
Although we have not identified strong RCT evidence to support the current role of oral isotretinoin in clinical practice, we have not identified any RCT evidence which challenges current practice. Due to the lack of evidence, some questions related to the use of oral isotretinoin for acne still need to be clarified in further studies that fulfil the following characteristics:
more robust sample sizes to allow: the observation of rare, but serious adverse effects; and subgroup analyses considering issues such as severity of acne (mild, moderate, and severe), affected areas (face or trunk, or both), age and gender;
longer duration and follow‐up to assess long‐term effects (benefits and harms);
standardisation of the best primary outcome, especially regarding the time points of outcome measurements; and
adherence to the CONSORT guideline providing recommendations for clinical trials in order to improve the quality of research and guide decision making (Schulz 2010).
With the aim of providing reliable physician guidelines and a robust evidence‐based support for daily clinical practice in acne therapy, future randomised clinical trials on oral isotretinoin for acne should focus on treatment of acne when there is insufficient response to therapy with oral antibiotics plus topical agents. To achieve more reliable results, trials should restrict their inclusion criteria to participants with moderate to severe acne from all ages (enabling subgroup analysis, such as assessment of very young adolescents), and genders; and assess the efficacy and safety of both therapies monthly during the treatment and for at least a one‐year follow‐up period. Future studies should be conducted in multiple settings, to ensure applicability of findings.
Assessment of different doses/regimens constitutes another important area of research related to oral isotretinoin for acne. In this case, randomised trials with a high number of participants presenting any degree of acne severity would be important to make clear the optimal dose/regimen, while taking into account safety concerns related to oral isotretinoin. RCTs on different doses/regimens of oral isotretinoin would also clarify issues regarding the best total cumulative dose according to acne severity.
Primary efficacy outcomes in further RCTs on oral isotretinoin must be based on the improvement of total inflammatory lesion counts, a more objective measure of clearing from acne than investigator's assessments of improvement measured by scales or by their subjective impressions. As acne has an important psychosocial impact, participant's assessment of the disease improvement and changes in quality of life should also be among efficacy outcomes in future trials on oral isotretinoin for acne. In addition, the nature and frequencies of adverse effects must be assessed and reported in a clear and pre‐standardised way, following directions from the CONSORT Statement (Ioannidis 2004; Schulz 2010). Also, the use of a uniform terminology in the evaluation and report of safety outcomes in acne trials, as recommended in MedDRA (Medical Dictionary for Regulatory Activities) (MedDRA 2016), should be adopted in future RCTs on oral isotretinoin for acne, as another effort to facilitate extraction, pooling, and replication of data from these studies. This applies, especially, to data related to less serious adverse effects of oral isotretinoin.
According to the recommendations of the CONSORT Statement (Schulz 2010), a clear description of the efforts to ensure random and concealed allocation of participants in further RCTs on oral isotretinoin for acne may reduce much of the downgrading of evidence found in our review. Also, an adequate and detailed report of all prespecified outcomes in the published protocol study can minimise the risk of reporting bias in future RCTs. The peculiar nature of mucocutaneous adverse effects related to oral isotretinoin use promotes difficulties in blinding of participants, personnel, and outcome assessors in clinical trials, a fact which is well recognised among acne researchers.
However, these concerns may not be used as a rationale for an absence of blinding of outcome assessment in further trials. Future technology, such as a specific computer software which makes the assessment of core acne outcomes more objective, may help (Min 2013). Also, future RCTs on acne should describe in detail all missing data, with the reasons for losses, besides adopting an adequate intention‐to‐treat analysis.
Nowadays, there are research groups working on the well recognized and well described issue of lack of standardised outcome measures and methodological practices in RCTs on acne therapy (Arowojolu 2012; Barbaric 2016; Cao 2015; Garner 2012). The Cochrane Skin Group Outcomes Research Initiative (CSG‐COUSIN, Schmitt 2016) and the Acne Core Outcomes Research Network (ACORN) (ACORN 2013) may guide acne outcome assessments in RCTs and help to increase the quality of the evidence from future research on acne therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2019 | Amended | Republished with edits for clarification |
Acknowledgements
The authors would like to thank for their methodological support: the Cochrane Skin Group editorial base; the Brazilian Cochrane Centre, the Brazilian Evidence‐based Critical Appraisal Group – BECA Group; and the Brazilian Cochrane Handbook Study Group.
The authors would like to thank the following colleagues from Cochrane for their prompt help with article translations: Esther J Van Zuuren (Dutch); Quan Yang (Chinese); Liv Merete Reinar (Norwegian); Katja Boehm (German); Kave Shams (Swedish).
The Cochrane Skin Group editorial base would like to thank the following people who commented on this review: our Key Editor, Robert Dellavalle; our Statistical Editor, Thomas Chu; our Methodological Editor, Ching‐Chi Chi; Miriam Santer and Gloria Sanclemente, who were the clinical referees; the consumer referee, who wishes to remain anonymous; and Nuala Livingstone from the Cochrane Editorial & Methods Department.
Appendices
Appendix 1. Skin Specialised Register (CRSW) search strategy
acne AND (isotretinoin or accutane or roaccutane or isotane or decutan or clarus or claravis or amnesteem or sotret or izotek or oratane or isotret or isoface or lurantal or isoacne or “13‐cis‐Retinoic Acid” or “Ro 4 3780” or “13 cis Retinoic Acid” or “Ro 4‐3780” or “Ro 43780” or “4759‐48‐2” or accure or aknenormin or ciscutan or isohexal or isotretinoin‐A or isosupra or isotroin or oratane or atretin or nimegen or acnotin or ruatine or sotret or tretin or roaccutan or roaccuttan or roacnetan or roacutan or roacuttan or "ro 04 3780" or acnal or acnetrex or akinol or curacne or curatane or “iso tretinoin” or “isoretinoic acid” or isoretinoin or isotren or isotrex or isotret‐hexal or newtinon or pinple or procuta or retinoin or “13 cis tretinoin”)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Acne Vulgaris explode all trees #2 (acne) #3 (#1 OR #2) #4 MeSH descriptor Isotretinoin explode all trees #5 isotretinoin or accutane or roaccutane or isotane or decutan or clarus or claravis or amnesteem or sotret or izotek or oratane or isotret or isoface or lurantal or isoacne or "13‐cis‐Retinoic Acid" or "Ro 4 3780" or "13 cis Retinoic Acid" or "Ro 4‐3780" or "Ro 43780" or "4759‐48‐2" or accure or aknenormin or ciscutan or isohexal or isotretinoin‐A or isosupra or isotroin or oratane or atretin or nimegen or acnotin or ruatine or sotret or tretin or roaccutan or roaccuttan or roacnetan or roacutan or roacuttan or "ro 04 3780" or acnal or acnetrex or akinol or curacne or curatane or "iso tretinoin" or "isoretinoic acid" or isoretinoin or isotren or isotrex or isotret‐hexal or newtinon or pinple or procuta or retinoin or "13 cis tretinoin" #6 (#4 OR #5) #7 (#3 AND #6)
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Acne Vulgaris/ 2. acne.mp. 3. 1 or 2 4. isotretinoin.mp. or exp ISOTRETINOIN/ 5. accutane.mp. 6. roaccutane.mp. 7. isotane.mp. 8. decutan.mp. 9. clarus.mp. 10. claravis.mp. 11. amnesteem.mp. 12. sotret.mp. 13. izotek.mp. 14. oratane.mp. 15. isotret.mp. 16. isoface.mp. 17. lurantal.mp. 18. isoacne.mp. 19. 13‐cis‐Retinoic Acid.mp. 20. Ro 4 3780.mp. 21. 13 cis Retinoic Acid.mp. 22. Ro 4‐3780.mp. 23. Ro 43780.mp. 24. 4759‐48‐2.rn. 25. accure.mp. 26. aknenormin.mp. 27. ciscutan.mp. 28. isohexal.mp. 29. isotretinoin‐A.mp. 30. isosupra.mp. 31. isotroin.mp. 32. oratane.mp. 33. atretin.mp. 34. nimegen.mp. 35. acnotin.mp. 36. ruatine.mp. 37. sotret.mp. 38. tretin.mp. 39. (roaccutan or roaccuttan or roacnetan or roacutan or roacuttan).mp. 40. "ro 04 3780".mp. 41. (acnal or acnetrex or akinol).mp. 42. (curacne or curatane).mp. 43. iso tretinoin.mp. 44. isoretinoic acid.mp. 45. (isoretinoin or isotren or isotrex or isotret‐hexal).mp. 46. newtinon.mp. 47. pinple.mp. 48. procuta.mp. 49. retinoin.mp. 50. 13 cis tretinoin.mp. 51. or/4‐50 52. 3 and 51 53. randomized controlled trial.pt. 54. controlled clinical trial.pt. 55. randomized.ab. 56. placebo.ab. 57. clinical trials as topic.sh. 58. randomly.ab. 59. trial.ti. 60. 53 or 54 or 55 or 56 or 57 or 58 or 59 61. (animals not (humans and animals)).sh. 62. 60 not 61 63. 52 and 62
Lines 53‐62: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision).
Appendix 4. Embase (Ovid) search strategy
1. exp Acne Vulgaris/ 2. acne.ti,ab. 3. 1 or 2 4. isotretinoin.mp. or exp ISOTRETINOIN/ 5. accutane.mp. 6. roaccutane.mp. 7. isotane.mp. 8. decutan.mp. 9. clarus.mp. 10. claravis.mp. 11. amnesteem.mp. 12. sotret.mp. 13. izotek.mp. 14. oratane.mp. 15. isotret.mp. 16. isoface.mp. 17. lurantal.mp. 18. isoacne.mp. 19. 13‐cis‐Retinoic Acid.mp. 20. Ro 4 3780.mp. 21. 13 cis Retinoic Acid.mp. 22. Ro 4‐3780.mp. 23. Ro 43780.mp. 24. 4759‐48‐2.rn. 25. accure.mp. 26. aknenormin.mp. 27. ciscutan.mp. 28. isohexal.mp. 29. isotretinoin‐A.mp. 30. isosupra.mp. 31. isotroin.mp. 32. oratane.mp. 33. atretin.mp. 34. nimegen.mp. 35. acnotin.mp. 36. ruatine.mp. 37. sotret.mp. 38. tretin.mp. 39. (roaccutan or roaccuttan or roacnetan or roacutan or roacuttan).mp. 40. "ro 04 3780".mp. 41. (acnal or acnetrex or akinol).mp. 42. (curacne or curatane).mp. 43. iso tretinoin.mp. 44. isoretinoic acid.mp. 45. (isoretinoin or isotren or isotrex or isotret‐hexal).mp. 46. newtinon.mp. 47. pinple.mp. 48. procuta.mp. 49. retinoin.mp. 50. 13 cis tretinoin.mp. 51. or/4‐50 52. crossover procedure.sh. 53. double‐blind procedure.sh. 54. single‐blind procedure.sh. 55. (crossover$ or cross over$).tw. 56. placebo$.tw. 57. (doubl$ adj blind$).tw. 58. allocat$.tw. 59. trial.ti. 60. randomized controlled trial.sh. 61. random$.tw. 62. or/52‐61 63. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 64. human/ or normal human/ 65. 63 and 64 66. 63 not 65 67. 62 not 66 68. 3 and 51 and 67
Appendix 5. PsycINFO (Ovid) search strategy
1. isotretinoin.mp. 2. accutane.mp. 3. roaccutane.mp. 4. isotane.mp. 5. decutan.mp. 6. clarus.mp. 7. claravis.mp. 8. amnesteem.mp. 9. sotret.mp. 10. izotek.mp. 11. oratane.mp. 12. isotret.mp. 13. isoface.mp. 14. lurantal.mp. 15. isoacne.mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 16. 13‐cis‐Retinoic Acid.mp. 17. Ro 4 3780.mp. 18. 13 cis Retinoic Acid.mp. 19. Ro 4‐3780.mp. 20. Ro 43780.mp. 21. accure.mp. 22. aknenormin.mp. 23. ciscutan.mp. 24. isohexal.mp. 25. isotretinoin‐A.mp. 26. isosupra.mp. 27. isotroin.mp. 28. oratane.mp. 29. atretin.mp. 30. nimegen.mp. 31. acnotin.mp. 32. ruatine.mp. 33. sotret.mp. 34. tretin.mp. 35. (roaccutan or roaccuttan or roacnetan or roacutan or roacuttan).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 36. "ro 04 3780".mp. 37. (acnal or acnetrex or akinol).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 38. (curacne or curatane).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 39. iso tretinoin.mp. 40. isoretinoic acid.mp. 41. (isoretinoin or isotren or isotrex or isotret‐hexal).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] 42. newtinon.mp. 43. pinple.mp. 44. procuta.mp. 45. retinoin.mp. 46. 13 cis tretinoin.mp. 47. acne.mp. 48. or/1‐46 49. 47 and 48 50. double‐blind.tw. 51. random$ assigned.tw. 52. control.tw. 53. 50 or 51 or 52 54. 49 and 53
Lines 50‐53: a therapy filter for PsycINFO (Ovid) created by the Health Information Research Unit at McMaster University.
Appendix 6. LILACS search strategy
acne and (isotretinoin or isotretinoina or accutane or roaccutane or isotane or decutan or clarus or claravis or amnesteem or sotret or izotek or oratane or isotret or isoface or lurantal or isoacne or accure or aknenormin or ciscutan or isohexal or isosupra or isotroin or oratane or atretin or nimegen or acnotin or ruatine or sotret or tretin or roaccutan or roaccuttan or roacnetan or roacutan or roacuttan or acnal or acnetrex or akinol or curacne or curatane or isoretinoin or isotren or isotrex or newtinon or pinple or procuta or retinoin)
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.
Appendix 7. MEDLINE (Ovid) adverse effects search strategy
1. exp *Isotretinoin/ae, ct, po, to [Adverse Effects, Contraindications, Poisoning, Toxicity] 2. accutane.ti,ab. 3. roaccutane.ti,ab. 4. isotane.ti,ab. 5. decutan.ti,ab. 6. clarus.ti,ab. 7. claravis.ti,ab. 8. amnesteem.ti,ab. 9. sotret.ti,ab. 10. izotek.ti,ab. 11. oratane.ti,ab. 12. isotret.ti,ab. 13. isoface.ti,ab. 14. lurantal.ti,ab. 15. isoacne.ti,ab. 16. 13‐cis‐Retinoic Acid.ti,ab. 17. Ro 4 3780.ti,ab. 18. 13 cis Retinoic Acid.ti,ab. 19. Ro 4‐3780.ti,ab. 20. Ro 43780.ti,ab. 21. 4759‐48‐2.ti,ab. 22. accure.ti,ab. 23. aknenormin.ti,ab. 24. ciscutan.ti,ab. 25. isohexal.ti,ab. 26. isotretinoin‐A.ti,ab. 27. isosupra.ti,ab. 28. isotroin.ti,ab. 29. oratane.ti,ab. 30. atretin.ti,ab. 31. nimegen.ti,ab. 32. acnotin.ti,ab. 33. ruatine.ti,ab. 34. sotret.ti,ab. 35. tretin.ti,ab. 36. (roaccutan or roaccuttan or roacnetan or roacutan or roacuttan).ti,ab. 37. "ro 04 3780".ti,ab. 38. (acnal or acnetrex or akinol).ti,ab. 39. (curacne or curatane).ti,ab. 40. iso tretinoin.ti,ab. 41. isoretinoic acid.ti,ab. 42. (isoretinoin or isotren or isotrex or isotret‐hexal).ti,ab. 43. newtinon.ti,ab. 44. pinple.ti,ab. 45. procuta.ti,ab. 46. retinoin.ti,ab. 47. 13 cis tretinoin.ti,ab. 48. exp *Isotretinoin/ 49. isotretinoin.ti,ab. 50. or/2‐49 51. exp product surveillance, postmarketing/ or exp adverse drug reaction reporting systems/ or exp clinical trials, phase iv/ 52. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab. 53. exp hypersensitivity/ or exp drug hypersensitivity/ or exp drug eruptions/ or exp hypersensitivity, delayed/ or exp hypersensitivity, immediate/ 54. exp anaphylaxis/ or exp conjunctivitis, allergic/ or exp dermatitis, atopic/ or exp food hypersensitivity/ or exp respiratory hypersensitivity/ or exp urticaria/ 55. side effect$.ti,ab. 56. exp Poisoning/ 57. exp hepatitis, toxic/ or exp hepatitis, chronic, drug‐induced/ 58. exp Substance‐Related Disorders/ 59. exp Drug Toxicity/ 60. exp Abnormalities, Drug‐Induced/ 61. exp Teratogens/ 62. exp Mutagens/ 63. exp Carcinogens/ 64. metabolite$.ti,ab. 65. exp dermatitis, contact/ or exp dermatitis, allergic contact/ or exp dermatitis, irritant/ or exp dermatitis, phototoxic/ 66. photoallergic reaction$.ti,ab. 67. exp dermatitis, allergic contact/ or exp dermatitis, photoallergic/ 68. phototoxicit$.ti,ab. 69. (sensitization or sensitisation).ti,ab. 70. exp Burning Mouth Syndrome/ 71. stinging.ti,ab. 72. burning.ti,ab. 73. fetal abnormalit$.ti,ab. 74. exp Drug Monitoring/ 75. drug effect$.ti,ab. 76. Sleep Apnea, Obstructive/ 77. ARRHYTHMIA/ 78. (safe or safety).ti,ab. 79. toxicity.ti,ab. 80. noxious.ti,ab. 81. complication$.ti,ab. 82. treatment emergent.ti,ab. 83. tolerability.ti,ab. 84. rebound.ti,ab. 85. Hypercalcemia/ci [Chemically Induced] 86. Urinary Calculi/ci [Chemically Induced] 87. Tachyphylaxis/ci, de [Chemically Induced, Drug Effects] 88. Substance Withdrawal Syndrome/ci, de [Chemically Induced, Drug Effects] 89. ATROPHY/ci [Chemically Induced] 90. TELANGIECTASIS/ci [Chemically Induced] 91. skin thinning.ti,ab. 92. Liver Diseases/ci [Chemically Induced] 93. Kidney Diseases/ci [Chemically Induced] 94. Disseminated Intravascular Coagulation/ci [Chemically Induced] 95. Multiple Organ Failure/ci [Chemically Induced] 96. Stevens‐Johnson Syndrome/ci [Chemically Induced] 97. Epidermal Necrolysis, Toxic/ci [Chemically Induced] 98. Heart Block/ci [Chemically Induced] 99. COMA/ci [Chemically Induced] 100. PARALYSIS/ci [Chemically Induced] 101. exp Nausea/ 102. exp Vomiting/ 103. benign intracranial hypertension.ti,ab. or exp Pseudotumor Cerebri/ 104. exp Pigmentation Disorders/ or pigmentation.ti,ab. or exp Pigmentation/ 105. lupus induced hepatitis.ti,ab. 106. suicide/ or suicidal ideation/ or suicide, attempted/ 107. suicide.ti,ab. 108. inflammatory bowel diseases/ or crohn disease/ 109. Colitis, Ulcerative/ 110. Anxiety/ 111. Depression/ 112. Mood Disorders/ 113. mood disturbance$.ti,ab. 114. (anxiety or depression or mood).ti,ab. 115. or/51‐114 116. 50 and 115 117. 1 or 116 The above strategy was combined with the cohort/case‐control filter from BMJ Clinical Evidence for MEDLINE, and limited to humans: 1. exp Cohort Studies/ 2. cohort$.tw. 3. controlled clinical trial.pt. 4. Epidemiologic Methods/ 5. limit 4 to yr=1966‐1989 6. exp case‐control studies/ 7. (case$ and control$).tw. 8. or/1‐3,5‐7
Appendix 8. Embase (Ovid) adverse effects search strategy
11. accutane.ti,ab. 2. roaccutane.ti,ab. 3. isotane.ti,ab. 4. decutan.ti,ab. 5. clarus.ti,ab. 6. claravis.ti,ab. 7. amnesteem.ti,ab. 8. sotret.ti,ab. 9. izotek.ti,ab. 10. oratane.ti,ab. 11. isotret.ti,ab. 12. isoface.ti,ab. 13. lurantal.ti,ab. 14. isoacne.ti,ab. 15. 13‐cis‐Retinoic Acid.ti,ab. 16. Ro 4 3780.ti,ab. 17. 13 cis Retinoic Acid.ti,ab. 18. Ro 4‐3780.ti,ab. 19. Ro 43780.ti,ab. 20. 4759‐48‐2.ti,ab. 21. accure.ti,ab. 22. aknenormin.ti,ab. 23. ciscutan.ti,ab. 24. isohexal.ti,ab. 25. isotretinoin‐A.ti,ab. 26. isosupra.ti,ab. 27. isotroin.ti,ab. 28. oratane.ti,ab. 29. atretin.ti,ab. 30. nimegen.ti,ab. 31. acnotin.ti,ab. 32. ruatine.ti,ab. 33. sotret.ti,ab. 34. tretin.ti,ab. 35. (roaccutan or roaccuttan or roacnetan or roacutan or roacuttan).ti,ab. 36. "ro 04 3780".ti,ab. 37. (acnal or acnetrex or akinol).ti,ab. 38. (curacne or curatane).ti,ab. 39. iso tretinoin.ti,ab. 40. isoretinoic acid.ti,ab. 41. (isoretinoin or isotren or isotrex or isotret‐hexal).ti,ab. 42. newtinon.ti,ab. 43. pinple.ti,ab. 44. procuta.ti,ab. 45. retinoin.ti,ab. 46. 13 cis tretinoin.ti,ab. 47. exp *Isotretinoin/ 48. isotretinoin.ti,ab. 49. or/1‐48 50. side effect$.ti,ab. 51. metabolite$.ti,ab. 52. photoallergic reaction$.ti,ab. 53. phototoxicit$.ti,ab. 54. (sensitization or sensitisation).ti,ab. 55. stinging.ti,ab. 56. burning.ti,ab. 57. fetal abnormalit$.ti,ab. 58. (toxic effect$ or drug effect$).ti,ab. 59. (safe or safety).ti,ab. 60. toxicity.ti,ab. 61. noxious.ti,ab. 62. complication$.ti,ab. 63. tolerability.ti,ab. 64. treatment emergent.ti,ab. 65. tolerability.ti,ab. 66. ((adverse or undesirable or harm$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab. 67. rebound.ti,ab. 68. skin thinning.ti,ab. 69. lupus induced hepatitis.ti,ab. 70. exp postmarketing surveillance/ 71. exp drug surveillance program/ 72. exp drug hypersensitivity/ or exp hypersensitivity reaction/ or exp delayed hypersensitivity/ or exp hypersensitivity/ or exp immediate type hypersensitivity/ 73. exp drug eruption/ 74. exp anaphylaxis/ 75. exp allergic conjunctivitis/ 76. exp atopic dermatitis/ 77. exp food allergy/ 78. exp respiratory tract allergy/ 79. exp urticaria/ 80. exp intoxication/ 81. exp toxic hepatitis/ 82. exp addiction/ 83. exp drug toxicity/ 84. exp teratogenic agent/ 85. exp mutagenic agent/ 86. exp carcinogen/ 87. exp contact dermatitis/ 88. exp skin allergy/ 89. exp irritant dermatitis/ 90. exp phototoxicity/ 91. exp photodermatosis/ or exp photoallergy/ 92. exp burning mouth syndrome/ 93. exp drug monitoring/ 94. exp sleep apnea syndrome/ 95. exp heart arrhythmia/ 96. hypercalcemia/ 97. urolithiasis/ 98. tachyphylaxis/ 99. withdrawal syndrome/ 100. atrophy/ 101. telangiectasia/ 102. liver disease/ 103. kidney disease/ 104. disseminated intravascular clotting/ 105. multiple organ failure/ 106. Stevens Johnson syndrome/ 107. toxic epidermal necrolysis/ 108. heart block/ 109. coma/ 110. paralysis/ 111. nausea/ 112. vomiting/ 113. benign intracranial hypertension.ti,ab. or exp brain pseudotumor/ 114. exp pigment disorder/ 115. exp pigmentation/ 116. pigmentation.ti,ab. 117. exp adverse drug reaction/ 118. exp drug safety/ 119. mood disorder/ 120. suicidal ideation/ 121. suicide.ti,ab. 122. suicide/ or suicide attempt/ 123. anxiety/ 124. depression/ 125. (anxiety or depression or mood).ti,ab. 126. enteritis/ 127. inflammatory bowel disease.ti,ab. 128. Crohn disease/ 129. ulcerative colitis/ 130. exp *isotretinoin/ae, to [Adverse Drug Reaction, Drug Toxicity] 131. or/50‐129 132. 49 and 131 133. 130 or 132 The above strategy was combined with the cohort/case‐control filter from BMJ Clinical Evidence for EMBASE and limited to humans: 1. exp cohort analysis/ 2. exp longitudinal study/ 3. exp prospective study/ 4. exp follow up/ 5. cohort$.tw. 6. exp case control study/ 7. (case$ and control$).tw. 8. or/1‐7
Data and analyses
Comparison 1. Oral isotretinoin versus any oral antibiotic plus any topical agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in acne severity assessed by a decrease in total inflammatory lesion count, measured in participants who were treated for a minimum period of 16 weeks | 3 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.96, 1.06] |
| 1.1 Oral isotretinoin versus oral minocycline plus azelaic acid cream | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.95, 1.05] |
| 1.2 Oral isotretinoin versus doxycycline plus adapalene/benzoyl peroxide gel | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.05] |
| 1.3 Oral isotretinoin versus tetracycline plus topical adapalene | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [0.50, 34.66] |
| 2 Frequency of serious adverse effects | 3 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.98] |
| 2.1 Oral isotretinoin versus oral minocycline plus azelaic acid cream | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Oral isotretinoin versus tetracycline plus topical adapalene | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Oral isotretinoin versus doxycycline plus adapalene/benzoyl peroxide gel | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.12, 72.98] |
| 3 Improvement in acne severity assessed by physician's global evaluation | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.00, 1.32] |
| 3.1 Very good or good improvement | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.97, 1.20] |
| 3.2 Decrease of at least two grades from baseline score | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.09, 1.38] |
| 4 Frequency of less serious adverse effects | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.42, 1.98] |
| 4.1 Four‐week analysis | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.03, 3.51] |
| 4.2 20‐week analysis | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.39, 1.97] |
| 5 Dropout rates | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Overall dropout rates | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.09] |
| 5.2 Dropout rates due to adverse effects | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.30, 2.31] |
| 5.3 Dropout rates due to no improvement or lack of efficacy | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 2.64] |
| 5.4 Dropout rates due to loss to follow‐up or not specified reasons | 3 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.26] |
Comparison 2. Oral isotretinoin versus oral isotretinoin plus any topical agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout rates | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.32, 3.10] |
Comparison 3. Standard oral isotretinoin versus other formulations of oral isotretinoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in acne severity assessed by physician's global evaluation | 2 | 1274 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.00, 1.11] |
| 2 Dropout rates | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Overall dropout rates | 2 | 1527 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.74, 1.34] |
| 2.2 Dropout rates due to adverse effects | 2 | 1527 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.43] |
| 2.3 Dropout rates due to loss to follow‐up, noncompliance or withdraw of consent | 2 | 1527 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2011.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 120 participants from a single centre, Department of Skin, STD, and Leprosy in SMS Medical College and Hospital, Jaipur ‐ India. Inclusion criteria: patients with mild (few to several papules/pustules with no nodule), moderate (several to many papules/pustules with few to several nodules), or severe acne vulgaris (numerous and/or extensive papules/pustules with many nodules). Exclusion criteria: pregnant females, married females desiring to get pregnant or using temporary methods of contraception, patients having family and/or personal history of hyperlipidaemia or diabetes, and those having drug‐induced acne. Age: for all participants, mean 18.95 years (range 14 ‐ 26, median 19) Gender: male ‐ 66 participants; female ‐ 46 participants Duration of acne: not provided Acne severity: mild ‐ 37 participants; moderate ‐ 38 participants; severe ‐ 37 participants |
|
| Interventions | Four different treatment regimens each consisting of 30 participants and lasting 16 weeks.
Along with oral isotretinoin, all participants were also given oral azithromycin 500 mg once a day 1 hour before meals for three days a week for three weeks. All participants were also advised to apply topical 1% clindamycin phosphate cream twice daily. |
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | Age, gender, and acne severity data were provided only for the whole 112 participants who completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by stratified randomisation method..." Comment: Despite the mention of stratified randomisation in the report, authors clarified by personal communication to us (email) that they actually adopted a simple (rather than stratified) randomisation process in each stratum (mild, moderate, and severe). Participants in each stratum picked one of the shuffled cards, which were in four different colours. Each one of the four available colours were linked to one of the four intervention groups |
| Allocation concealment (selection bias) | Low risk | Comment: By personal communication to us, authors described a pharmacy‐controlled randomisation (an independent pharmacy prepared the drug containers, which were of identical appearance for all interventions groups, as were the capsules, all from the same brand). It was likely the allocation was concealed via the randomisation method used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: By personal communication to us, authors described an open design. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: By personal communication to us, authors described an open design, with no blinding of outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 120 patients with a mean age of 18.95 years (range 14 ‐ 26, median 19) were included in the present prospective study... For final result analysis, there were 112 patients as described in the flowchart." Comment: The level of loss to follow‐up could be a source of bias. Furthermore, the trial reported a 'per‐protocol' analysis, which did not consider data from loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section. |
| Other bias | High risk | Quote: "Along with oral isotretinoin, all patients were also given oral azithromycin 500 mg once a day 1 hour before meals for three days a week for three weeks. All the patients were also advised to apply topical 1% clindamycin phosphate cream twice daily." Comment: There was a high risk of bias due to an inappropriate administration of a co‐intervention. Concomitant treatment with an oral antibiotic plus a topical antibiotic applied to all four intervention groups might underestimate the potential higher risk of acne flare (an adverse effect) on the first weeks of therapy among participants on higher isotretinoin doses. |
Ahmad 2015.
| Methods | Parallel design, randomised clinical trial | |
| Participants | 58 participants from a single centre, Department of Dermatology and Venereology, Faculty of Medicine, Minia University, Minia, Egypt Inclusion criteria: participants stopped any acne treatment 1 week before starting oral isotretinoin Exclusion criteria: female participants who are or might become pregnant during treatment, lactating mothers, participants with pre‐existing liver disease, abnormal liver function test, or hyperlipidaemia, participants with acne conglobata and acne fulminans Age: 12 to 39 years (median 20 years; mean and SD of 22.12 ± 7.05) Gender: 15 males, 43 females Duration of acne: not available Acne severity: mild (6), moderate (13), severe (31), very severe (11) |
|
| Interventions | All participants received oral isotretinoin at a dose of 0.5 – 1.0 mg/kg/day. During the initial month of treatment, only half of the anticipated maintenance dose was given to avoid acne exacerbation, as previously recommended.
The median starting dose during the first month of treatment was 30 mg/day (mean = 28.4 ± 4.8), whereas the median maintenance dose thereafter was 60 mg/day (mean = 55.6 ± 10.9). Participants received a median total cumulative dose of 126.1 mg/kg body weight (mean = 126 ± 22.5) and the median duration of treatment was 22 weeks (mean = 21.40 ± 3.73). |
|
| Outcomes |
Serum cholesterol, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were evaluated before and 3 months after starting treatment. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | No information available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: The trial did not provide any information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The study compared single versus divided daily dose of oral isotretinoin and did not use a placebo for blinding purpose |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: The study compared single versus divided daily dose of oral isotretinoin and did not use a placebo for blinding purpose |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no losses |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: No other risk of bias was found |
Akman 2007.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 66 participants from multiple centres in Turkey (number of centres was not stated) Inclusion criteria: acne with the FDA global grade 2 (moderate) and 3–4 (severe); participants with acne who had not responded to conventional antibiotic therapy or who had rapidly relapsed after conventional treatment Exclusion criteria: acne conglobata, acne fulminans, or systemic disorders requiring any treatment Age: by group, mean ± standard deviation (years).
Gender: 23 male/37 female
Duration of acne: not provided Acne severity: 29 moderate/31 severe
|
|
| Interventions |
|
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Akdeniz University Scientific Research Projects Unit | |
| Notes | Age, gender, and acne severity data were provided only for participants who completed the study in each group. Besides this, there was a difference in the numerical data of males and females in each group between the results section of the main text and Table 1 presented in the report. Authors provided the correct data to us by personal communication (email). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "This was a multicenter, randomised and controlled study consisting of 66 patients... Six patients failed to continue the follow‐up." Comment: The level of loss to follow‐up could lead to a considerable attrition bias. Furthermore, the trial presented a 'per‐protocol' analysis, which did not consider data from loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Corlin 1984.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 191 participants from multiple centres (14 Departments of Dermatology) in Germany Inclusion criteria: diagnosis of severe papulopustular acne which was untreatable with ordinary methods Exclusion criteria: not stated Age: 21 years (mean) – range 14 to 42 years Gender: 148 male/43 female Duration of acne: not provided Acne severity: all participants had severe papulopustular acne |
|
| Interventions |
All three interventions were given for 20 weeks. |
|
| Outcomes |
All clinical outcome measurements were recorded at baseline, every 2 weeks and, after 3 months, every 4 weeks until the end of the treatment. Laboratory parameters were assessed at baseline, 4, 12, and 20 weeks. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Hoffmann‐La Roche AG | |
| Notes | Both reports of this study were in German and data were extracted by a German member of the Cochrane Collaboration indicated by Cochrane Skin Group One author worked at Hoffmann‐La Roche AG, Grenzach‐Wyhlen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The study had an open design, with participants and personnel aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study had an open design. However, there was no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: The trial reported a 'per‐protocol' analysis for clinical efficacy outcomes. All randomised participants (191) were considered in the analysis of laboratory alterations and adverse effects. However there was a considerable level of losses to follow‐up, which could underestimate differences regarding adverse effects between groups, as participants who were lost‐to follow up earlier could have a higher number of adverse effects if they had received the interventions during the whole period of therapy (20 weeks). |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Cumurcu 2009.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 51 participants from a single centre, Department of Dermatology, Gaziosmanpasa, a University School of Medicine in Turkey Inclusion criteria: participants with acne vulgaris who had laboratory and routine physical examination results within the normal limits Exclusion criteria: systemic hypertension; coronary arterial disease; familial hyperlipidaemia; diabetes mellitus; renal or hepatic functional disorders; severe osteoporosis or severe pulmonary, gastrointestinal, or hematologic problems; having dry eyes, intolerance to contact lenses, or clinical blepharoconjunctivitis during the pretreatment ophthalmologic assessment Age: mean ± standard deviation (years)
Gender: 20 male/31 female
Duration of acne: not provided Acne severity: not provided |
|
| Interventions |
Duration of treatment not stated |
|
| Outcomes |
All outcomes were measured at baseline, at days 45 and 90 of treatment, and at follow‐up, 30 days after the end of treatment, but subjective ocular complaints were detected at the end of the trial and at follow‐up * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Forty‐nine of the 51 patients completed the study. Two patients dropped out of the study: one patient in Group 1 because of blurred vision, and one patient in Group 2 for unrelated reasons". Comment: Analysis included those lost to follow‐up during the study and incomplete outcome data were balanced in numbers across groups. However, there was no balance in reasons for missing outcomes across intervention groups and this could be a source of bias, as all outcomes of the trial had a very low frequency. |
| Selective reporting (reporting bias) | High risk | Quote: "Subjective complaints (photophobia, burning, itching, scratching) were significantly higher in Group 1 compared with baseline (P < 0.05). However, 1 month after the discontinuation of treatment, the difference between the groups was not significant (P > 0.05)". Comment: No protocol available and one outcome, subjective complaints, was reported incompletely, so that it could not be entered in a meta‐analysis (only the P value was reported). |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
De 2011.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 41 participants from Postgraduate Institute of Medical Education and Research, Chandigarh, India.Inclusion criteria: participants with severe grade 4 acne according to the FDA global score Exclusion criteria: not provided Age: not provided Gender: not provided Duration of acne: not provided Acne severity: severe |
|
| Interventions |
Participants in both groups received interventions for eight months |
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | Only a preliminary report of this study was available as a conference proceeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: The high level of loss to follow‐up in the study could lead to a considerable attrition bias (33% in Group A and 45% in Group B) |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Dhaked 2016.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 240 participants from a single centre, the outpatient clinic in the dermatology department of SMS Medical College, Jaipur ‐ India Inclusion criteria: patients with moderate (several to many papules/pustules with few to several nodules) and severe acne vulgaris (numerous and/or extensive papules/pustules with many nodules), involving all body sites Exclusion criteria: pregnant women, women desiring to get pregnant or using temporary methods of contraception, patients having family and/or personal history of hyperlipidaemia or diabetes, and those having drug‐induced acne. Age: for all participants, mean 18.88 years (standard deviation 2.46, range 15‐30, median 18.51) Gender: male ‐ 189 participants; female ‐ 45 participants Duration of acne: < 1 year ‐ 66 participants; 1 to 3 years ‐ 113 participants; > 3 years ‐ 55 participants Acne severity: moderate ‐ 118 participants; severe ‐ 116 participants |
|
| Interventions |
All participants were also advised to apply topical 1% clindamycin phosphate cream twice daily and white petroleum jelly on lips, when required |
|
| Outcomes |
Authors evaluated adverse effects, total acne load (TAL), and treatment response according to mean percentage decrease in TAL at an interval of: two weeks, during the 24‐week therapy; six weeks, for the 12 weeks after completion of treatment. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None | |
| Notes | Age, gender, and acne severity data were provided only for the whole 112 participants who completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Out of 240 patients, six patients were lost to follow up during the study period. For final result analysis, there were 234 patients (Group A, 118 and Group B, 116)." Comment: The trial reported a 'per‐protocol' analysis, which did not consider data from loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Dhir 2008.
| Methods | Parallel design, randomised controlled trial Duration of trial: from November 2004 to April 2006 |
|
| Participants | 60 participants from a single centre, the Department of Dermatology, Indian Naval Health Service (INHS) Asvini, Mumbai, India Inclusion criteria: clinically diagnosed cases of nodulocystic acne Exclusion criteria: being pregnant or breastfeeding; having abnormal lipid profiles, significant hepatic dysfunction, or an underlying psychiatric disorder Age: 81.5% (49) participants: 16‐25 years Gender: 44 males/16 females
Duration of acne: not provided Acne severity: all included participants had nodulocystic acne |
|
| Interventions |
|
|
| Outcomes |
Participants were examined every four weeks. Clinical scoring and side effects were recorded at each visit during both therapy phase and follow‐up period (six months). * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | Age data were not presented as means or a closed range for each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is an open label..." Comment: Participants and personnel were not blinded; they were aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This is an open label..." Comment: The study had an open design. However, there was no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Out of the 60 patients who were included in the study, 50 completed the treatment while five patients dropped out from each group." Comment: The trial reported a 'per‐protocol' analysis, which did not consider data from loss to follow‐up. Also, the report did not detail reasons for missing data. |
| Selective reporting (reporting bias) | High risk | Quote: "Patients were examined every four weeks and clinical scoring and side effects were recorded at each visit. This was supplemented by the physician’s global assessment as..." Comment: No protocol available and a selective choice of data for an outcome might have occurred, since physician’s global assessment of improvement in acne severity was performed every four weeks, during the therapy phase, but only data from the assessment at visit 6 (24 weeks, end of treatment) were reported. |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Faghihi 2014.
| Methods | Parallel design, randomised controlled trial Duration of the trial: from September 2012 until September 2013 |
|
| Participants | 58 participants from a single dermatologic clinic, located at Al‐Zahra General Hospital, Isfahan, Iran Inclusion criteria: having moderate to severe facial acne vulgaris Exclusion criteria: acne secondary to other problems; pregnancy or intention of pregnancy; breastfeeding; other dermatological diseases of the face; G6PD deficiency; history of having taking any medication that could interact with dapsone (e.g. trimethoprim‐sulfametoxazol) or isotretinoin (e.g. tetracyclines, methotrexate and vitamin A supplements) within the previous 3 months; and known hypersensitivity to the study medication Age: for all participants, ranged from 18 to 25 years Age: by group, mean ± standard deviation (years)
Gender: 25 male/33 female
Duration of acne: 3.19 (mean) ± 1.8 (standard deviation) years; 2 to 5 years (range) Acne severity: moderate to severe |
|
| Interventions | Group A (n = 29): oral isotretinoin, 20 mg once a day plus 5% dapsone gel applied on the face twice daily, both for 8 weeks Group B (n = 29): oral isotretinoin, 20 mg once a day plus vehicle neutral gel applied on the face twice daily, both for 8 weeks |
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "During the study period, only a caregiver who was not involved in the experiment was aware of the contents of the tubes; the patients and the examiner were blind to the topical compounds." Comment: There was a description of who was masked during the conduct of the trial, despite no description of an evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "During the study period, only a caregiver who was not involved in the experiment was aware of the contents of the tubes; the patients and the examiner were blind to the topical compounds." Comment: There was an explicit report of blinding of outcome assessment, despite having not reported an evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...and all the participants completed the study." Comment: As there were no missing data, the authors had probably performed a real intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: There was an available protocol on a clinical trials registry and all outcomes listed in methods section of the protocol were adequately reported in the final publication |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Farrell 1980.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 16 participants from a single centre, the Department of Dermatology, University of Iowa College of Medicine, Iowa City, United States of America Inclusion criteria: severe acne vulgaris with refractoriness to conventional therapy; and presence of a minimum of ten or more deep cystic nodules, each having a greatest diameter of 4 mm or more Exclusion criteria: abnormal laboratory profile Age: 16 to 31 years Gender: male Duration of acne: not provided Acne severity: severe |
|
| Interventions |
All interventions were given for 12 weeks |
|
| Outcomes |
The three above outcomes were assessed weekly for the first 8 weeks of treatment and then at 2‐week intervals for the last 4 weeks of treatment and for an 8‐week post treatment period. Laboratory assessments (haemogram, urinalysis, and blood chemistries) evaluated at baseline, 2, 4, 8, and 12 weeks of therapy and at 8 weeks after therapy was discontinued*. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Hoffman‐La Roche Inc., and a research grant (RO‐1‐AM‐22083‐02) from the United States Public Health Service | |
| Notes | The number of participants stated for each intervention group refers to participants who completed the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to a treatment schedule according to a computer‐generated randomised code..." Comment: The trial described an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...in a double‐blind study." "...the drug was dispensed by an individual not involved in patients evaluation. Therefore, neither the physicians involved in the clinical evaluation nor the patients were aware of individual assignment to the three dose groups". "At the end of the study when the code was broken...". Comment: There was a description of who was masked during the conduct of the trial, despite no description of any evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Therefore, neither the physicians involved in the clinical evaluation nor the patients were aware of individual assignment to the three dose groups". Comment: This statement did not clarify whether all outcome assessors were blinded. It was not clear in the report if all the outcome assessments and the clinical evaluation of participants were done by the same people from the study staff. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Fourteen of the sixteen patients completed the study according to protocol." "As stated earlier, this patient was then dropped from the double‐blind study, and no further data from this case are included in sub‐sequent weeks." Comment: The trial reported a 'per‐protocol' analysis, which did not consider data from loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Goldstein 1982.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 56 participants from multiple centres, three Departments of Dermatology, in United States of America Inclusion criteria: male participants with severe, treatment‐resistant nodulocystic acne; good general health; and to have at least ten inflammatory acne nodules or cysts, 4 mm or more in diameter, on the face, back, or chest Exclusion criteria: not stated Age: for all participants, ranged from 14 to 54 years (mean: 23.4 years) Gender: 100% male Duration of acne: average period of 8 years (range: 2 to 26 years) Acne severity: severe |
|
| Interventions |
Both drugs were administered in two divided doses daily, for 8 weeks |
|
| Outcomes |
The three outcomes above were assessed at each visit and performed at baseline, 2, 4, and 8 weeks of therapy and at 4 and 8 weeks post‐therapy Laboratory alterations assessed by: urinalysis, complete blood count, and blood chemistry determinations repeated after 1, 2, and 8 weeks of therapy; semen analyses, including determination of sperm count, motility, and morphology, performed at baseline and the end of therapy and 8 weeks later* Frequency of ophthalmologic alterations assessed by examination, including dilated slit‐lamp and determination of ocular tension, done on each participant at baseline and after completion of drug therapy* * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A multicenter double‐blind study..." "The capsules were coded and dispensed in such a fashion that neither the patients nor the examining physician knew which retinoid was being administered". Comment: There was a description of who was masked during the conduct of the trial, despite no description of any evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...neither the patients nor the examining physician knew which retinoid was being administered". Comment: This statement did not clarify whether all outcome assessors were blinded. It was not clear in the report if all the outcome assessments and the clinical evaluation of participants were done by the same people from the study staff |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "All fifty‐six patients completed the 8‐week course of drug therapy, and fifty‐one of these completed the 8‐week follow‐up period." Comment: The report did not provide detailed numbers and reasons for missing data in each intervention group |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Gollnick 2001.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 85 participants from 10 centres in Germany, Austria and Switzerland Inclusion criteria:
Exclusion criteria: women, participants with milder (comedonal or papulopustular acne) or more severe (acne fulminans, acne tetrade) forms of acne, photosensitive participants, and participants with contraindications to isotretinoin or minocycline, and those hypersensitive to the excipients contained in the azelaic acid cream Age: for all participants, mean/range (years): 19/15 ‐ 31
Gender: 100% male Duration of acne: mean/range (years): 4/0 ‐ 14
Acne severity: 100% severe |
|
| Interventions | AA/Mino group (n = 50):
Iso group (n = 35):
|
|
| Outcomes | Primary efficacy outcome:
Secondary efficacy outcomes:
Other outcomes:
Patient examinations were done for all participants at baseline and at monthly intervals over the 6‐month treatment period in study phase 1. In study phase 2, participants of the initial AA/mino group were examined at monthly intervals over the 3‐month maintenance treatment period, but the participants of the initial Iso group were examined only once (after completion of the second 3‐month study phase). * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...the study design was essentially open label..." Comment: Participants and personnel were not blinded; they were aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...the study design was essentially open label..." Comment: The study had an open design. However, there was no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 85 eligible patients recruited to the study, 77 completed the first study phase (90.6%). Eight patients (6 from the AA/Mino group, 2 from the Iso group) dropped out of the study because of poor compliance, adverse reactions, lack of efficacy, infringement of the study protocol or for other reasons. All 85 patients were included in the analysis of the efficacy. " Comment: The level of loss to follow‐up could lead to a considerable attrition bias. Reasons for attrition were described, but there was imbalance of missing data between intervention groups |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available; data from participants subjective global assessment of improvement after therapy were not reported, despite having been listed in the methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Jones 1983a.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 76 participants from two centres, Departments of Dermatology from the General Infirmary and from St James’s University Hospital, both at Leeds, United Kingdom Inclusion criteria: failure to respond to long‐term oral antibiotic therapy; partial response to antibiotics, but rapid relapse on cessation of such therapy Exclusion criteria: not stated Age: mean ± standard deviation (years)
Gender: 45 male/31 female
Duration of acne: not provided Acne severity: moderate to severe |
|
| Interventions |
All interventions were administered for 16 weeks |
|
| Outcomes |
Outcomes were estimated at all visits: initial, at 4‐week intervals during 16 weeks, and at 32 weeks (after the follow‐up period). * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Roche Products Limited | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A double‐blind dose‐response study on seventy‐six patients is now reported..." Comment: There was no description of who was blinded and also no description of efforts to ensure blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A double‐blind dose‐response study on seventy‐six patients is now reported..." Comment: There was no description of who was blinded and also no effort to ensure blinding was described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Thirteen patients (nine relapses, four failures) received further treatment prior to the 32 weeks and were withdraw from the study at that point. Five patients failed to attend during the follow‐up period for a variety of reasons." "...the drug schedule had to be altered in only three patients. These three patients were excluded from the study." Comment: There was no clear description of missing data for each group in the report. However, the level of loss to follow‐up could lead to a considerable attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Jones 1983b.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 90 participants from Leeds Dermatological Research Foundation, Department of Dermatology, The General Infirmary at Leeds, United Kingdom Inclusion criteria: participants with moderate to moderately severe acne Exclusion criteria: not stated Age: not provided Gender: not provided Duration of acne: not provided Acne severity: moderate to moderately severe |
|
| Interventions |
Participants received isotretinoin for 16 weeks and erythromycin for 24 weeks |
|
| Outcomes |
Assessments occurred every 4 ‐ 8 weeks; follow‐up continued every 8 ‐ 12 weeks off therapy. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Ninety patients have been studied and randomly allocated on a double‐blind basis..." "The appropriate placebos were given in the first two groups." Comment: There was a description of an appropriate method to ensure blinding, despite not having described clearly who was blinded, or any evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Ninety patients have been studied and randomly allocated on a double‐blind basis..." "The appropriate placebos were given in the first two groups." Comment: There was a description of an appropriate method to ensure blinding, despite not having described clearly who was blinded, or any evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The report did not provide detailed numbers and reasons for missing data in each intervention group |
| Selective reporting (reporting bias) | High risk | Quote: "The group receiving antibiotic alone showed less improvement (50%) than the other two groups (70 %) at the end of the treatment period. The combination therapy showed no significant difference from the 13‐cis‐retinoic group alone." Comment: There was no clearly and detailed report of the number of participants enrolled in each group. Also, authors did not describe which type of efficacy outcome measurement (acne grade or facial lesion count) had the particular difference in acne improvement cited in the report |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Kapadia 2005.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 60 participants from 3 medical centres, in Karachi, Pakistan Inclusion criteria: not provided Exclusion criteria: not provided Age: not provided Gender: not provided Duration of acne: not provided Acne severity: moderate to severe |
|
| Interventions |
Interventions were administered for 24 weeks |
|
| Outcomes |
Assessments were done at baseline and every 8 weeks till 24 weeks. Laboratorial investigations were performed at baseline and at the end of the study. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “None of the patients were lost to follow‐ up” Comment: An intention‐to‐treat analysis was probably done |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available and not all detected adverse effects had frequencies reported separately for each intervention group |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
King 1982.
| Methods | Parallel design; randomised controlled trial | |
| Participants | 48 participants from two centres, Departments of Dermatology from the General Infirmary and from St James’s University Hospital, both at Leeds, United Kingdom Inclusion criteria: failure to respond to conventional therapy, including several courses of antibiotics Exclusion criteria: not stated Age: for all participants, mean age was 25 years Gender: 24 male/24 female Duration of acne: not provided Acne severity: not provided |
|
| Interventions |
All interventions were administered for 16 weeks |
|
| Outcomes |
|
|
| Funding body | Roche Products Limited | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A double‐blind study of the effects of 13‐cis‐retinoic acid on acne, sebum excretion rate and microbial population". Comment: There was no description of who was blinded and also no description of efforts to ensure blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A double‐blind study of the effects of 13‐cis‐retinoic acid on acne, sebum excretion rate and microbial population". Comment: There was no description of who was blinded and also no description of efforts to ensure blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no information regarding missing data |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Lee 2011.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 60 participants from a single centre, the Department of Dermatology, Chung‐Ang University Hospital, Seoul, Korea Inclusion criteria: moderate acne which had not responded to antibiotic therapy or which had rapidly relapsed after antibiotic therapy Exclusion criteria: severe acne types, such as conglobata or fulminans; other systemic diseases; history of oral isotretinoin or oral contraceptive use previously or concurrently; pregnancy and lactation; and history of other acne treatment in the preceding 3 months Age: all participants, ranged from 16 to 33 years Age: by group, mean ± standard deviation (years)
Gender: 20 male/40 female Duration of acne: mean ± standard deviation (years)
Acne severity: moderate acne |
|
| Interventions |
Interventions were administered for 24 weeks |
|
| Outcomes | Improvement in acne severity assessed by:
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | A Chung‐Ang University Research grant in 2010 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to treatment groups in a 1 : 1 : 1 ratio using a computer‐generated randomisation schedule." Comment: The trial described an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was not possible to blind either the patient or the therapist, but the examiner was blinded to group assignment during collection of the data." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “This was a 24‐week, prospective, randomised, controlled, open with blinded assessment trial”. "It was not possible to blind either the patient or the therapist, but the examiner was blinded to group assignment during collection of the data." Comment: There was a clear description of blinded outcome assessment during the conduct of the trial, despite no description of any evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Analyses were done according to intention‐to‐treat principle..." Comment: Despite having cited the term "intention‐to‐treat", the study presented a level of loss to follow‐up (20%, 15%, and 20%, respectively, in Groups A, B and C) which could have led to a considerable attrition bias. Reasons for attrition were described and there was a imbalance between intervention groups. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Leheta 2011.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 60 participants from Faculty of Medicine, Cairo University, Cairo, Egypt and Faculty of Medicine, Alexandria University, Alexandria, Egypt Inclusion criteria: participants with mild to moderate acne Exclusion criteria: not provided Age: not provided Gender: not provided Duration of acne: not provided Acne severity: mild to moderate acne |
|
| Interventions |
There was no description of the duration of therapy phase for groups A and B, or of the follow‐up period for all groups |
|
| Outcomes |
Time points of outcome measurement not provided * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Selective reporting (reporting bias) | High risk | Quote: "However, there was no statistically significant difference (P = 0.401) between the treatment groups, as regards the acne severity score after treatment, ..." Comment: There was no clearly and detailed report of the number of participants enrolled in each group. Also, the only exact numerical data provided in the results section of the study regarding the comparison between the three intervention groups was the P value cited above. |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Lester 1985.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 30 participants from two centres, Departments of Dermatology of Sunnybrook Medical Center and Women’s College Hospital, at Toronto, Canada Inclusion criteria: presence of 10 or more deep dermal inflamed cystic nodules, which measured 4 mm or more on their longest diameter; past of minimal responses to treatment with tetracycline; failure to respond to other oral and topic agents, including antibiotics, topical tretinoin, benzoyl peroxide, and prednisone, as well as many other conventional forms of acne therapy. Exclusion criteria: pregnant and women of childbearing potential; significant ocular, hepatic, renal, or hematologic diseases or abnormalities; superficial x‐ray therapy within 6 months prior to the study; history of hypersensitivity to tetracycline or to vitamin A and its derivatives Age: for all participants, ranged from 17.1 to 37.7 years Age: by group, mean ± standard deviation/range (years)
Gender: male/female
Duration of acne: mean ± standard deviation (years)/range
Acne severity: 100% severe |
|
| Interventions |
|
|
| Outcomes | Clinical efficacy assessments:
The ophthalmic examination was done at the end of the treatment period and spermatogenesis evaluations were performed at 12, 20, and 24 weeks. All other outcomes measurements were made at 2, 4, 8, 12, and 16 weeks during drug administration, and at 20 and 24 weeks during the follow‐up period. * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Hoffman‐La Roche Ltd | |
| Notes | Authors declared being from the Clinical Research Unit of Hoffman‐La Roche Ltd., Toronto, Ontario, Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The double‐blind condition of the trial was preserved by the pharmacists, who dispensed the appropriate test medication at the dosage level prescribed by the investigator. In addition, the objective cyst evaluations were performed by a research nurse independent of consultation with the investigator ”. Comment: It was not clear if the term "double‐blind" was a reference to participants and personnel or to investigators (personnel) and independent research nurse (outcome assessor) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “...the objective cyst evaluations were performed by a research nurse independent of consultation with the investigator”. Comment: There was no description of blinding assessment for all outcomes reported in the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Two patients in the tetracycline‐treatment group withdrew from the study prematurely due to poor control of their disease". Comment: There was an imbalance in missing outcome data due to ‘inefficacy’ across the two intervention groups |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Oprica 2007.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 52 participants from a single centre, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden Inclusion criteria: male and female participants who had moderate or severe inflammatory acne vulgaris (at least grade 3 on the face, back, or chest according to the Leeds technique); age range 15–35 years Exclusion criteria: papulo‐pustular acne with no nodules or very severe forms (acne fulminans); use of oral/topical acne treatments within 8 weeks of the start of treatment; use of drugs that may interfere with tetracycline (i.e. retinoids, anticoagulants, antacids, iron preparations, hepatic enzyme inducers); pregnant women or those who, wanted to become pregnant; breastfeeding mothers; systemic or psychiatric diseases (including drug and alcohol abuse); any dermatological condition that might interfere with the evaluation of acne; acne due to secondary causes; participation in any other clinical trial; hypersensitivity or allergy to the study medication Age: for all participants, ranged from 15 to 35 years Age: by group, median ± SD (years)
Gender: male/female
Disease duration: median ± SD (years):
Acne severity: moderate or severe acne |
|
| Interventions |
|
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Edward Welander and Finsen Foundations; F. Hoffman‐La Roche Ltd Co | |
| Notes | There was no report of demographic data for all initially randomised participants (52) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly to one of 2 treatment groups, using a computer‐generated randomisation code..." Comment: The trial described an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "...randomisation code known only to a person not involved in the trial." Comment: There was an adequate concealment of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "In our open study, both treatments improved the clinical condition". Comment: Participants and personnel were not blinded; they were aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In our open study, both treatments improved the clinical condition". Comment: The study had an open design. However, there was no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "A total of 52 patients were randomised; 26 to receive TET/ADA and 26 to receive ISO. Of these, 3 patients abandoned the study after randomisation and were not included in further analyses. Overall, 7 patients in the ISO group and 6 patients in the TET/ADA group abandoned the study at different times. The therapy was regarded as completed due to a very good clinical response before the end of 6 months in one patient from the ISO group." "Analysis of clinical efficacy parameters was performed on the intention‐to‐treat population, which included all patients who had at least one post‐baseline evaluation. For patients who prematurely discontinued the treatment, the last observations were carried forward." Comment: Despite having cited the term "intention‐to‐treat", actually authors performed a "per protocol" analysis, since analysis did not included data from all initially randomised participants |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in methods section |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was sponsored and promoted by a pharmaceutical company |
Peck 1982.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 33 participants from a single centre, the Clinical Center of the National Institutes of Health, at Bethesda, United States Inclusion criteria: having at least ten inflamed deep derma or subcutaneous acne cysts or nodules of at least 4 mm diameter; history of minimal response to treatment with oral and topical antibiotics, oral vitamin A, topical vitamin A acid, topical benzoyl peroxide, x‐irradiation, oral contraceptives, oral dapsone, intralesional injections of corticosteroids, oral prednisone, surgical drainage, applications of liquid nitrogen, photochemotherapy with psoralen and long wave ultraviolet light, and other acne therapies Exclusion criteria: pregnant women and fertile women who refused to use birth control measures Age, gender and duration of acne: not provided Acne severity: not stated if moderate or severe, only reported as cystic acne |
|
| Interventions |
At the monthly visit, "if there had been no change or only a slight worsening of the acne, then the dose of isotretinoin or placebo was increased by 0.5 mg/kg/day. If there had been a marked worsening, the protocol permitted a cross‐over to active drug if the patient had been taking placebo." |
|
| Outcomes |
Clinical and laboratory side effects evaluated at baseline and monthly intervals during and after treatment* * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Hoffman‐La Roche Inc. | |
| Notes | We considered for our review only the first four weeks of the study: after this time point, switching of participants from placebo group to isotretinoin group had started. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned... according to a computer‐generated code." Comment: The trial described an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “If there had been a marked worsening, according to predetermined criteria, then the double‐blind code was broken.” "...or an equivalent number of placebo capsules identical in appearance. The capsules were dispensed to the patients by a third party ..." Comment: Despite having described efforts to keep blinding of participants and personnel during the first study phase ("double‐blind"), there was no explicit reporting of who was and was not blinded across participants, personnel, and outcome assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: It was not clear in the report whether the person who had done the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: An intention‐to‐treat analysis was probably done, as there was no dropout for the first four weeks of the study which were considered for our analysis (after this time point, switching of participants from placebo group to isotretinoin group had started) |
| Selective reporting (reporting bias) | High risk | Quote: “In four matched pairs, the average sebum production was 0.25 mg lipids/10 cm2/3 hr, with a range of 0.19 to 0.35, taken from 12 to 16 weeks on therapy”. Comment: No protocol available and the report in the results section suggested that not all participants from each group had been assessed for one outcome (reduction in sebum production); selective choice of subsets of data for this outcome, which was listed in the methods section, might have occurred |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as a pharmaceutical company promoted it |
Pigatto 1986.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 24 participants from two departments (2nd Department of Dermatology and 3rd Department of Medicine) of only one centre, the School of Medicine, University of Milan, Italy Inclusion criteria: male participants with severe cystic acne vulgaris Exclusion criteria: overweight; people assumed to be consuming more than 5 g of alcohol daily or smoking more than 15 cigarettes daily; use of any drug which could have interfered with lipid metabolism; abnormal liver function tests and altered glucose tolerance tests Age: for all participants, mean age was 23 years (standard deviation ± 3 years), with range from 20 to 29 years Gender: all participants were male Duration of acne: not provided Acne severity: severe cystic acne |
|
| Interventions |
|
|
| Outcomes |
Laboratory analysis: hematology, blood chemistry, and urinalysis were measured at baseline, monthly, and at change of doses* Laboratory parameters of the lipid metabolism assessed at baseline, monthly, and at change of doses* . * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no mention to missing data in the report |
| Selective reporting (reporting bias) | High risk | Quote: "Laboratory examinations were done monthly and at the change of doses." Comment: No protocol available. Measurements of lipid metabolism parameters were reported only at baseline, 10 weeks, and 20 weeks: selective choice of data for this outcome might have occurred |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Prendiville 1988.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 40 participants from two centres: Ealing Hospital at Middlesex and St John's Hospital for Diseases of the Skin at London, United Kingdom Inclusion criteria: not provided Exclusion criteria: not provided Age: for all participants, ranged from 16 to 31 years Gender: 100% male Duration of acne: not provided Acne severity: severe |
|
| Interventions |
Interventions were administered for 16 weeks |
|
| Outcomes |
Assessments for all outcomes were made at 0, 4, 8, 16, 20, 28, and 36 weeks Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Roche Products Limited | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “In view of the mucocutaneous side‐effects of 13‐cis retinoic acid which make double‐blind assessment of this drug impossible, our study was conducted on a single‐blind basis”. “Each slide was graded blind using the acne grading scale (0‐10)”. Comment: Participants and personnel were not blinded; they were aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "...our study was conducted on a single‐blind basis.” “Each slide was graded blind using the acne grading scale (0‐10)”. Comment: The blinding of outcome assessment was clearly stated only for one of the five outcomes of the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Six patients failed to complete the study, three in each group. Four patients, one on dapsone and three on 13‐cis retinoic acid were withdrawn because of non‐attendance or failure to take medication as directed". Comment: Reasons for attrition were described and there was imbalance of missing data between intervention groups |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available. Besides this, one outcome, subjective improvement assessed by each participant by a visual analogue scale, was not reported using the measures prespecified on the methods section (only P values at each time point of measurement were reported) |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was promoted by a pharmaceutical company |
Rademaker 2013b.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 60 participants from three private dermatological practices in metropolitan New Zealand Inclusion criteria: participants from both gender, aged 25‐55 years of age, with low‐grade adult acne, which was defined as three or more acne lesions month on the face, for at least three months Exclusion criteria: having acne greater than grade 2, by the Modified Leeds Acne Assessment Scale (Burke 1984); pregnancy (or unwilling to use contraception methods), breast‐feeding, any significant systemic illness, BMI over 35, use of any systemic medication likely to influence the participant's acne (including systemic glucocorticoids or antibiotics), use of any topical or systemic anti‐acne products in the preceding four weeks; oestrogen and/or progesterone therapy (including levonorgestrel‐releasing intrauterine device) which was not on a stable dose for at least 6 months before the start of the study; being on a systemic retinoid in the preceding 6 months Age: for all participants, ranged from 25 to 55 Age: by group, mean ± standard deviation (years)
Gender: 8 males; 52 female Duration of acne: not provided Acne severity: low‐grade |
|
| Interventions |
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Douglas Pharmaceuticals | |
| Notes | Despite having enrolled 60 participants, the main report of this study provided demographic data only for 58 participants, which investigators considered as the 'intention to treat population' The main author, Rademaker M, declared being on the speaker bureau for Douglas Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following written informed consent, patients were randomised to receive either isotretinoin for 32 weeks..." Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This investigator initiated, industry‐sponsored study was designed, as a randomised, double‐blind, placebo‐controlled, parallel group clinical study..." "The placebo capsules were developed to be indistinguishable in smell, taste and appearance from the 5 mg isotretinoin test product." Comment: There was a description of blinding of participants and personnel during the conduct of the trial, despite no description of any evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To keep the assessor blinded to adverse effects (e.g. dry lips), the DLQI, patient diary and safety assessments were performed by a study nurse separately to the acne lesion count and erythema scoring." Comment: There was an explicit report of blinding of outcome assessment, despite not having reported an evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Protocol deviations required exclusion of 12 patients from the PP evaluation at week 16..." Comment: Despite having performed both per protocol and last‐observation‐carried‐forward techniques to analyse data, the high level of missing data at all time points of measurement suggested that an unbiased analysis was unlikely |
| Selective reporting (reporting bias) | Low risk | Comment: There was an available protocol on a clinical trials registry and all outcomes listed in the methods section of the protocol were adequately reported in the final publication |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was promoted by a pharmaceutical company |
Shetti 2013.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 100 participants attending Outpatient Department of Dermatology, District hospital, Mandya Inclusion criteria: participants of either gender, more than 18 years, with moderate to severe acne vulgaris Exclusion criteria: pregnant and lactating women. Age: more than 18 years Gender: of either gender Duration of acne: not provided Acne severity: moderate to severe |
|
| Interventions |
Daily dose per kilogram and duration of therapy ‐ not provided |
|
| Outcomes |
Time points of outcome measurements not clearly provided ‐ authors just cited regular time intervals of assessment * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | Data above were limited, as we first found only a conference proceeding related to this study. We evaluated a full‐text reference in a late stage of our review, after the latest update searches. Additional data from this newer report did not impact conclusions of this review at the late stage; we just incorporated the reference as secondary citation for the study in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "An open labeled randomised prospective controlled study of moderate to severe acne vulgaris receiving oral isotretinoin in low dose continuous and low dose intermittent regimen was conducted. " Comment: Participants and personnel were not blinded; they were aware of the treatment arm to which participants were allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "An open labeled randomised prospective controlled study of moderate to severe acne vulgaris receiving oral isotretinoin in low dose continuous and low dose intermittent regimen was conducted. " Comment: The study had an open design. There was no information regarding blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Selective reporting (reporting bias) | High risk | Quote: "There was statistically significant difference in GAGS score between group A and group B (P < 0.005). " "Side effects were more frequent with low dose continuous isotretinoin than low dose intermittent isotretinoin regimen". "There was a statistically significant elevation of LDL levels (P < 0.001) in low dose continuous isotretinoin regimen". Comment: There was no detailed and accurate report of numerical data in the results section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Strauss 1984.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 150 participants from three medical centres in United States Inclusion criteria: severe, treatment‐resistant, nodulocystic acne and a minimum of ten inflammatory nodulocystic acne lesions at least 4 mm in diameter on the face, back, and chest Exclusion criteria: not provided Age: by group, mean age (years)
Gender: 127 male/14 female
Duration of acne: mean (years)
Acne severity: severe |
|
| Interventions |
Interventions were administered for 20 weeks, but therapy could be stopped when a 70% to 80% reduction in the number of lesions had been obtained |
|
| Outcomes |
Incidence of laboratory abnormalities assessed by tests done at baseline, 4, 8 weeks and at the end of treatment * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | There was no report of demographic data for all initially randomised participants (150) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment of individual dosing schedule was done by a randomised, computer generated code..." Comment: The trial described an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was conducted in a double‐blind fashion, so that the investigator doing the lesion counts did not know the dosage of drug for any individual". Comment: It was unclear if, besides the investigator, participants and other personnel were also blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study was conducted in a double‐blind fashion, so that the investigator doing the lesion counts did not know the dosage of drug for any individual". Comment: The blinding of outcome assessment was clearly stated only for one outcome of the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Data from a few of the patients was not complete and could not be used in the final analysis of the drug trial, but at least forty‐six patients were used in the analysis of each group." Comment: Loss to follow‐up data were somewhat different in the two reports of the study, but even the minor level of loss to follow‐up reported in the primary reference of the study could lead to a considerable attrition bias. Also, the number of withdrawals was different between intervention groups |
| Selective reporting (reporting bias) | High risk | Quote: “Data for week 2 of isotretinoin treatment have not been presented because the number of patients who returned at that time was relatively small”. Comment: No protocol available and selective choice of data for an outcome probably occurred, as week 2 was a predefined time point at the methods section of the main report of the study |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Strauss 2001.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 602 participants from multiple centres (17) in United States Inclusion criteria: males or non‐pregnant; non‐nursing females 12 years of age or older; severe recalcitrant nodular acne, with 10 or more nodular lesions (facial and truncal) at least 5 mm in diameter Exclusion criteria: use of vitamin A supplements in excess; sensitivity or allergy to parabens. hypersensitivity to vitamin A or its derivatives; use of tetracyclines within 3 days of starting the treatment; recent history of drug or alcohol abuse; any skin disease that might interfere with the evaluation of acne; baseline symptoms of significant depression assessed as severe by the Beck Depression Inventory‐II (BDI‐II); any previous treatment with etretinate (Tegison) or acitretin (Soriatane); treatment with standard isotretinoin within the previous 180 days; treatment with standard isotretinoin before the last 180 days that was associated with a serious adverse event including significant depression or insomnia to a degree that affected the ability to work or perform daily activities; weight less than 30 kg or greater than 130 kg; and any clinically significant elevation of laboratory values at screening Age: for all participants ('per protocol population'), ranged from 12 to 46 years Age: by group, mean ± SD/median/range (years)
Gender: male/female
Duration of acne: not provided Acne severity: severe |
|
| Interventions |
Both interventions administered for 20 weeks |
|
| Outcomes | Primary efficacy outcome:
Secondary efficacy outcome:
Safety outcomes:
Safety assessments were made at weeks 0, 2, 4, 8, 12, 16, 20. Laboratory tests were performed during the screening/baseline and either at week 20 or at the end of therapy * Indicates outcomes which matched those prespecified for this review. |
|
| Funding body | A grant from Roche Laboratories Inc. | |
| Notes | There was no report of demographic data for all initially randomised participants (602) Some authors declared being consultants or employees from Roche Laboratories, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization numbers were based on a 1:1 randomisation and distributed in a block size of two". Comment: The trial did not describe an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “A comparative, double‐blind, double‐dummy, multicenter efficacy study of 602 patients…” “Investigators were blinded as to randomisation number generation”. Comment: Despite the description of efforts to keep the double‐blind design, it was not clear who was blinded. The trial did not report if outcome assessments and clinical evaluation/treatment prescription were done by the same people from the study staff |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Investigators were blinded as to randomisation number generation”. Comment: Despite the description of efforts to keep the double‐blind design, it was not clear who was blinded. The trial did not report if outcome assessments and clinical evaluation/treatment prescription were done by the same people from the study staff |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (efficacy outcomes): “The Per Protocol analysis population was defined in the study protocol as patients who were randomised and received at least 12 weeks of the study medication, did not have any major protocol violations, had 80% compliance with the allocated treatment, and had not used Ortho Tri‐Cyclen as a method of contraception”. Comment (efficacy outcomes): The study adopted a ‘per protocol’ analysis. From 602 participants initially randomised to each treatment group, 110 (51 originally allocated in group 1 and 59, in group 2) were not included in the analysis in the groups to which they were randomised. This level of loss to follow‐up could lead to a considerable attrition bias Quote (safety outcomes): "All patients who took at least one dose (intent‐to‐treat [ITT] population) of micronised isotretinoin or standard isotretinoin were included in the safety analysis. " Comment (safety outcomes): In the efficacy report of the same study, a high level of loss to follow‐up was stated, making it unlikely that a real ITT analysis for safety outcomes had been performed. The measurement of the effect of differences regarding adverse effects between groups might have been underestimated, since participants who were lost‐to follow up earlier could have a higher number of adverse effects if they had received the interventions during the whole period of therapy (20 weeks) |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available and probably there was a selective choice of data for outcomes. According to the methods section of the efficacy report, lesion counts and participants'/investigators' assessments of global response were done at baseline, 8, 16, and 20 weeks, but only changes between measurements at baseline and at the end of the therapy (at 12 to 20 weeks) were reported |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was promoted by a pharmaceutical company |
Tan 2014.
| Methods | Parallel design, randomised controlled trial Duration of the trial: from November 2011 to July 2013 |
|
| Participants | 266 subjects from 29 centres in Canada Inclusion criteria: male or female subject of any race, aged 12 to 35 years inclusive; subject weighing between 50 and 110 kg; subject with severe acne (IGA at least 4), which in the opinion of the investigator is appropriate for treatment with oral isotretinoin (severe nodular acne, severe inflammatory acne, recalcitrant acne; all unresponsive to conventional first‐line therapies); subject with at least 5 nodules on the face Exclusion criteria: subject with clinically abnormal results to blood tests performed at screening; subject with acne conglobata, acne fulminans, secondary acne (chloracne, drug‐induced acne, etc.), pyoderma faciale, sinus tracks; female subject who is pregnant, nursing or planning a pregnancy during the study; subject with known history of hepatic and/or renal insufficiency, to be confirmed by blood tests; subject with known metabolic or structural bone disease (for 12‐17 years old population); subject with bowel disease and/or with hypervitaminosis A; subject who presents with treated or untreated depression or has a history of depression including a family history of major depression; subject with a wash‐out period (from baseline for topical treatment on the face) of less than two weeks (corticosteroids, antibiotics, antibacterials, antiseptics, retinoids, other anti‐inflammatory drugs or other acne treatments), one week (cosmetic procedures), three months (photodynamic therapy and laser therapy for acne); subject with a wash‐out period from baseline for systemic treatment of less than: corticosteroids, antibiotics (4 weeks), progesterone for contraception (3 months); spironolactone (3 months), other acne treatments (6 months), cyproterone acetate (6 months). Age: for all participants, ranged from 12 to 35 years Age: by group, mean ± standard deviation (years)
Gender:
‐ Duration of acne: not provided ‐ Acne severity: severe |
|
| Interventions |
|
|
| Outcomes | Efficacy outcomes:
Safety outcomes:
Composite efficacy/safety outcome:
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Galderma | |
| Notes | Authors declared being advisor, consultant, investigator, speaker, or employee of a company with commercial interest in the results of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Prior to the start of the study, the randomisation list was generated by a statistician. The RANUNI routine of the Statistical Analysis System (SAS; Institute Inc., Cary, NC) was used for the kit number generation. Subjects were randomised in a 1:1 ratio for each group." Comment: The study adopted an adequate method to generate the random sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation list was secured in a locked cabinet and in an electronic file with restricted access to only the designated personnel directly responsible for labelling and handling the study treatments..." Comment: There was an adequate concealment of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This phase IIIb, non‐inferiority, multicentre, randomised, investigator‐blinded, controlled, parallel group study recruited subjects of any race". Comment: The authors reported clearly that participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Investigators did not have access to the randomisation list and study treatments were dispensed by the designated study drug dispenser – someone other than the investigator/rater. Both study drug dispenser and subject were instructed not to discuss the study treatments with the investigator/rater." Comment: There was an explicit report of blinding of outcome assessment, despite not having reported an evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Three study populations were analysed: safety, intent‐to‐treat (ITT), and per‐protocol (PP) populations. The last observation carried forward method was used to impute missing efficacy values. For the composite endpoint, missing values were considered as unsuccessful." Comment: Since missing data were not equally distributed across groups (regarding both number of missing subjects and reasons for incomplete outcome data), it was unlikely that data analysis was unbiased, even with the use of well recognised methods of data imputation |
| Selective reporting (reporting bias) | Low risk | Comment: There was an available protocol on a clinical trials registry and outcomes listed in the methods section of the protocol were adequately reported in the final publication |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was promoted by a pharmaceutical company |
Van der Meeren 1983.
| Methods | Parallel design, randomised controlled trial | |
| Participants | 58 participants from five Departments of Dermatology of University Hospitals, in the Netherlands Inclusion criteria: male participants with therapy‐resistant acne conglobata and multiple inflamed or hemorrhagic cysts or nodules Exclusion criteria: hypertriglyceridaemia; women (because of teratogenic effects in animal experiments) Age: mean ± standard deviation (22 ± 6) years, range 15 to 31 Gender: 100% male Duration of acne: mean ± standard deviation (7 ± 5) years Acne severity: severe |
|
| Interventions |
Both groups received the interventions for 12 weeks. After this time point, the dose of oral isotretinoin in both groups could be reduced or increased, but no more than 1.0 mg/kg/daily, until completing 24 weeks of treatment |
|
| Outcomes |
Laboratory assessments were done at baseline, 2, 4, 12, and 24 weeks. All other outcomes were evaluated at each visit, which occurred every 2 weeks * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: The trial did not provide any information about random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no statement regarding methods of allocation concealment in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study did not provide any information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Dry lips and dry skin made the continuation of therapy impossible in 5 patients, 4 of group I and only 1 patient of group II. " Comment: Reasons for attrition were completely described in the Dutch report of the study, and there was an imbalance of missing data and reasons for loss to follow‐up across intervention groups during the first 12 weeks of the trial, which we considered in our review |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in the methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Wahab 2008.
| Methods | Parallel design, randomised controlled trial Duration of the trial: from January, 2004 to May, 2007 |
|
| Participants | 60 participants from three medical centres in Dhaka, Bangladesh Inclusion criteria: cases having moderate to severe acne as categorised by the Global Acne Grading Score, GAGS Exclusion criteria: not provided Age: for all participants, range from 15 to 30 years Age: by group, mean ± standard deviation (years)
Gender: male/female
Duration of acne: mean ± standard deviation (months)
Acne severity: moderate/severe
|
|
| Interventions |
|
|
| Outcomes |
There were monthly assessments of the outcomes during the treatment phase * Indicates outcomes which matched those prespecified for this review |
|
| Funding body | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: The report did not provide any information about random sequence generation. However, after being contacted by email, an author said that they used drawing of lots |
| Allocation concealment (selection bias) | High risk | Comment: There was no statement regarding methods of allocation concealment in the study. However, after being contacted by email, an author said that no concealment method was used while allocating interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The study did not provide any information to permit judgement. However, after being contacted by email, an author said that no method was used to ensure blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: The study did not provide any information to permit judgement. However, after being contacted by email, an author said that no method was used to ensure blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was no statement regarding loss to follow‐up or exclusions in the report. However, after being contacted by email, an author said that there were no missing data |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available; however, there was an adequate report of outcomes listed in the methods section |
| Other bias | Low risk | Comment: There were no other apparent sources of bias |
Webster 2014.
| Methods | Parallel design, randomised controlled trial Duration of the trial: from September, 2009 to October, 2011 |
|
| Participants | 925 participants from 49 medical study centres in USA and Canada Inclusion criteria: severe recalcitrant nodular acne, compatible with oral isotretinoin use; 10 or more nodular lesions (including face and trunk); no prior exposure to systemic isotretinoin or other retinoids; age between 12 and 54 years; weight between 40 and 110 kg Exclusion criteria: pregnancy or high risk of becoming pregnant; breastfeeding or considering breastfeeding during the course of the treatment; skin diseases that could interfere with the evaluation of the study medications; concurrent or previous gastrointestinal disease; known suicidal behaviour; psychosis or psychotic symptoms; carcinoma; liver or kidney disease; pseudotumour cerebri; rheumatoid arthritis or vitamin D depletion disease, and paediatric participants with serum 25‐hydroxyvitamin D levels < 20 ng /ml Age: for all participants, ranged from 12 to 52 years Age: by group, mean ± standard deviation (years)
Gender: male/female
‐ Duration of acne: not provided ‐ Acne severity: severe |
|
| Interventions |
|
|
| Outcomes | Primary efficacy outcomes: both assessed through lesion counts:
Secondary efficacy outcome:
Exploratory efficacy outcome:
Safety assessments:
* Indicates outcomes which matched those prespecified for this review |
|
| Funding body | Cipher Pharmaceuticals Inc. | |
| Notes | All authors declared being consultants or employees from a manufacturer of oral isotretinoin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A blocked randomisation regimen, not revealed to the sponsor or investigators, provided an approximately balanced allocation to the 2 treatment groups. Patients were randomised in a 1:1 ration to receive a 30‐day supply". Comment: Despite the description of a blocked randomisation process, the trial did not describe an adequate method for the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "At screening, patients were assigned to receive 1 of the 2 treatment regimens using a centralized randomised system prior to the first dose of medication." Comment: Authors described an adequate method of allocation sequence concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both formulations were over‐encapsulated for blinding purposes and were identically packaged. Treatment assignment was concealed to patients, investigators, their staff, and the clinical research team". Comment: There was a description of both who was masked during the conduct of the trial and efforts to keep blinding, despite no description of any evaluation of the success of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment assignment was concealed to patients, investigators, their staff, and the clinical research team". Comment: There was a clear description of blinded outcome assessment during the conduct of the trial, despite no description of any evaluation of the success of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Since missing data were not equally distributed across groups (regarding both number of missing subjects and reasons for incomplete outcome data), it was unlikely that data analysis was unbiased, even with the use of well recognised methods of data imputation |
| Selective reporting (reporting bias) | Low risk | Comment: There was an available registered protocol and the study final report described all pre‐specified outcomes according to the protocol text |
| Other bias | Unclear risk | Comment: The study might be at risk of inappropriate influence of funders, as it was promoted by a pharmaceutical company |
AA: azelaic acid ADA: adapalene ALT: alanine aminotransferase AST: aspartate aminotransferase BDI‐II: Beck Depression Inventory‐II BMI: Body Mass Index BUT: break‐up time test D+A/BPO: doxycycline plus adapalene/benzoyl peroxide DEXA: dual energy x‐ray absorptiometry DLQI: Dermatology Life Quality Index FDA: US Food and Drug Administration G6PD: Glucose‐6‐ phosphate dehydrogenase GAAS: Global Acne Assessment Scale GAGS:global acne grading system score GAS: Global acne scoring IGA: Investigator global assessment ISO: isotretinoin kg: kilogram LDL: low‐density lipoproteins MDI/ICD‐10: Major Depression Inventory mg: miligram mm: millimetre PAE: Patient Assessment of Efficacy PGSA: Physician's Global Severity Assessment QoL: Quality of life SD: standard deviation SMS: Sawai Man Singh STD: Sexually transmitted disease TAL: total acne load TCA: trichloroacetic acid TET: tetracycline VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Herane 2009 | This RCT studied a gel hydrating cream to manage dryness of the skin related to oral isotretinoin therapy. |
| Kus 2005 | This RCT studied vitamin E to manage dryness of the skin related to oral isotretinoin therapy. |
| Li 2004 | This RCT had an inappropriate comparator intervention (an alternative treatment, Qingfei Liangxue Fa, which is a prescription from traditional Chinese medicine that is used for clearing the lungs and removing fat). |
| Lin 1999 | This RCT included participants with another condition, haemodialysis‐related acne, and not acne vulgaris. |
| Liu 2008 | This RCT had an inappropriate comparator intervention (an alternative treatment: encircling acupuncture combined with venesection and cupping). |
| Shen 2000 | This RCT had an inappropriate comparator intervention (an alternative treatment: compound shecao decoction, from traditional Chinese medicine). |
| Strauss 2000 | This RCT had tested another intervention: vitamin E. |
| Sun 2000 | This RCT had an inappropriate comparator intervention (an alternative treatment: An Ti Shu Tong, 20 mg twice daily, from traditional Chinese medicine). |
| Williams 1992 | This RCT had tested another intervention: intranasal mupirocin. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Faghihi 2017.
| Methods | Parallel design, randomised controlled trial Duration of the trial: from 2014 to 2015 |
| Participants | 66* participants from 1 single study centre at Isfahan, Iran Inclusion criteria: patient consent to participate in the study, no sensitivity to retinoids, no pregnancy, not willing to become pregnant, and absence of hormonal disorders in patients Exclusion criteria: failure of the patient to attend follow‐up sessions for any reason, and adoption of other supplementary therapies during the study Age: by group, mean ± standard deviation (years)
Gender: male/female
Duration of acne: not provided Acne severity: moderate to severe |
| Interventions |
|
| Outcomes |
* Indicates outcomes which matched those prespecified for this review |
| Notes | * Authors cited 60 participants in the abstract section. However, when authors referred to numbers of males and females in each group in the results section of the report, it was clear that there were 66 participants. Authors did not clearly report if there was loss to follow‐up. |
GAGS: Global Acne Grading System VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
IRCT201104094310N6.
| Trial name or title | Comparison of therapeutic effects of oral erythromycin along with low dose oral Isotretinoin and oral erythromycin and low dose flutamide versus doxycyclin in female severe acne vulgaris |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: females with severe acne Exclusion criteria: pregnancy; breastfeeding; liver disease; hyperlipidaemia; receiving anti‐acne drugs; OCP pills; spironolactone in the last 2 months Age: no age limit Gender: female |
| Interventions |
|
| Outcomes |
Time point: onset of study and days 30 and 60 Method of measurement: observation |
| Starting date | 20 September 2013 |
| Contact information | m.golmohammadi@arums.ac.ir |
| Notes |
IRCT2013110315246N1.
| Trial name or title | Acne cure rate in patients hyperandrogenism with two drugs of decuttane and cyptroterone compound |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: female; age between 14‐45 years old; besides acne, hirsutism (Ferriman & Gallwey score greater than or equal to 4) and Ludwing grade one or higher female androgenetic alopecia; absence of contraindications to treatment with tablets decuttane and cyproterone compound based on questionnaire; not planning a pregnancy or breast‐feeding; individual participants signed a consent form Exclusion criteria: drug allergy; pregnancy or lactation; uncooperative patient or patient's consent to continue reading |
| Interventions |
|
| Outcomes |
|
| Starting date | 18 April 2012 |
| Contact information | kioumars_jamshidi@med.mui.ac.ir |
| Notes |
ASI: acne severity index BID: twice (two times) a day OCP: oral contraceptive Tcc: triclocarban TLC: Total Acne Lesion Count
Differences between protocol and review
Electronic searches:
We intended to search for nonrandomised controlled trials (nonRCTs) (case‐control and cohort) on adverse effects of oral isotretinoin for acne only in MEDLINE, but we carried out an additional search in Embase. We had also planned to use the Skin Group's standard adverse effects search strategy and the disease and intervention terms, but actually used an amended version and did not search on disease. Both deviations from our original plans were due to our intention of undertaking a very comprehensive search on adverse effects of oral isotretinoin, a drug associated with alarming safety concerns.
Compared with the published protocol, there were some alterations in the tasks completed by review authors: CSC, EB and RR assessed the titles and abstracts of studies retrieved in the search rather than just CSC and EB.
Type of interventions:
We have included studies analysing oral isotretinoin versus oral isotretinoin plus systemic or topical treatments, as these combinations of anti‐acne drugs may be seen in clinical daily practice (Zaenglein 2016). This comparison was not in our inclusion criteria during protocol phase.
Types of outcomes measures:
In the review, we clarified what the definition of a serious adverse event was: "We classified an adverse effect as serious if it: was fatal; life threatening; permanently disabling; or required hospitalisation."
Types of outcomes measures:
Some of the outcomes were measured at different time points. To make reporting easier, we classified outcome measurements into short‐ and long‐term.
Measures of treatment effect:
In our protocol, we had planned to use both risk ratio and risk difference to measure dichotomous outcomes. Instead of this, we decided to report only risk ratios, as there is empirical evidence of increased consistency in relative effect measures than in absolute effect measures (Deeks 2002; Engels 2000).
Assessment of reporting biases:
We could not use funnel plots to assess reporting biases, as originally planned, once we established that none of our meta‐analyses had included more than three studies (Higgins 2011).
Data synthesis:
Instead of not pooling data from studies when significant methodological or clinical heterogeneity were detected (I² value higher than 50%), we decided to pool data using the random‐effects model and explore the possible reasons for heterogeneity. Further, we kept the narrative approach to report measures of effect from each study separately, as we had stated in our protocol. When there was no statistical heterogeneity from the chi‐square test (I2), we applied the random‐effects model to pool data (instead of the fixed‐effects model, as we had previously planned).
Subgroup analysis and investigation of heterogeneity:
We found a huge diversity of intervention characteristics among each one of our eligible trials, especially regarding comparisons of different doses and therapeutic regimens of oral isotretinoin. Subgroup analyses that considered severity of acne, treatment duration, degree of improvement in acne severity, age, and gender, as we planned to conduct at the protocol stage, were not feasible due to scarce data and small number of studies in each meta‐analysis.
Sensitivity analysis:
We could not carry out a sensitivity analysis of results from our included RCTs as we intended by excluding trials of low and moderate risk of bias (we referred to this as methodological quality at the time the protocol was written), due to the scarcity of studies and data in each one of our analyses of effects.
'Summary of findings' table and GRADE:
We had not planned to include a 'Summary of findings' ('SoF') table and the GRADE system (Guyatt 2011) to evaluate the quality of evidence in our review at the time the protocol was published. However, following the Cochrane Skin Group's recommendations, we added this table to summarise the outcomes for the main comparison, oral isotretinoin versus oral antibiotics plus topical agents. The GRADE system was applied to assess the quality of the whole body of evidence for each outcome presented in this review.
Contributions of authors
CSC, RR and EB are the contact persons within the editorial base. CSC and RR coordinated contributions from the co‐authors. CSC, RR and ALCM wrote the final draft of the review. CSC, EB, RR, ALCM screened papers against eligibility criteria. CSC and EB obtained data on ongoing and unpublished studies. CSC, EB, RR, ALCM appraised the quality of papers. CSC, EB, RR, ALCM extracted data for the review and sought additional information about papers. CSC entered data into RevMan. CSC, EB, EKS and RR analysed and interpreted data. CSC and RR worked on the methods sections. CSC, EB and PM drafted the clinical sections of the background and responded to the clinical comments of the referees. CSC, RR and ALCM responded to the methodology and statistics comments of the referees. MML was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. CSC, RR, ALCM, EB, EMKS and PM are the guarantors of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil.
Support in the form of salary to Ediléia Bagatin, Edina MK da Silva, and Rachel Riera
External sources
-
Centro de Referência e Treinamento DST/AIDS ‐ Governo do Estado de São Paulo, São Paulo, Brazil.
Support in the form of salary to Caroline S Costa
-
Congregação Associação Santa Catarina, São Paulo, Brazil.
Support in the form of salary to Marília M Lúcio
-
University of Newcastle, Callaghan, Australia.
Support in the form of salary to Parker Magin
-
General Practice Training Valley to Coast, Australia.
Support in the form of salary to Parker Magin
-
Elermore Vale General Practice, Australia.
Support in the form of salary to Parker Magin
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
-
Universidade Federal do Piauí, UFPI ‐ Teresina, Piauí, Brazil.
Support in the form of salary to Caroline S Costa
Declarations of interest
Caroline S Costa: I received a grant from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (Capes), fundacao do Ministerio da Educacao (MEC), a Brazilian government granting agency. Ediléia Bagatin: I received money from Bayer for: board membership for studies and research on adult female acne; for expert testimony on work for guidelines of treatment for adult female acne; for lectures in the Continuing Medical Education Program on adult female acne; and support for participation in the International Congress of Dermatology to present work on adult female acne. Ana Luiza C Martimbianco: none known Edina MK da Silva: none known Marília M Lúcio: none known Parker Magin: none known Rachel Riera: none known
Edited (no change to conclusions)
References
References to studies included in this review
Agarwal 2011 {published and unpublished data}
- Agarwal US. Randomization process, allocation concealment and blinding used in the trial [personal communication]. E mail to: C Costa 6 August 2013.
- Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: a randomized comparative trial. Indian Journal of Dermatology, Venereology and Leprology 2011;77(6):688‐94. [CENTRAL: CN‐00835026] [DOI] [PubMed] [Google Scholar]
Ahmad 2015 {published data only (unpublished sought but not used)}
- Ahmad HM. Analysis of clinical efficacy, side effects, and laboratory changes among patients with acne vulgaris receiving single versus twice daily dose of oral isotretinoin. Dermatologic Therapy 2015;28(3):151‐7. [CENTRAL: CN‐01076629] [DOI] [PubMed] [Google Scholar]
Akman 2007 {published and unpublished data}
- Akman A, Durusoy C, Senturk M, Koc CK, Soyturk D, Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: a randomized, controlled multicenter study. Archives of Dermatological Research 2007;299(10):467‐73. [CENTRAL: CN‐00627669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corlin 1984 {published data only (unpublished sought but not used)}
- Corlin R, Maas B, Mack‐Hennes A. Oral administration of low doses of 13‐cis‐retinoic acid in acne papulopustulosa. Results of a multicenter study [13‐cis‐Retinsäure. Niedrig dosiert orale Anwendung bei Acne papulopustulosa]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 1984;35(12):623‐9. [CENTRAL: CN‐00175184] [PubMed] [Google Scholar]
Cumurcu 2009 {published data only (unpublished sought but not used)}
- Cumurcu T, Sezer E, Kilic R, Bulut Y. Comparison of dose‐related ocular side effects during systemic isotretinoin administration. European Journal of Ophthalmology 2009;19(2):196‐200. [CENTRAL: CN‐00682992] [DOI] [PubMed] [Google Scholar]
De 2011 {published data only}
- De D, Kanwar AJ. Standard‐dose isotretinoin vs. a combination of low‐dose isotretinoin and azithromycin pulse in management of severe acne: preliminary report of a randomized study. British Journal of Dermatology 2011;165(Suppl 1):41. [CENTRAL: CN‐00843694; EMBASE: 70479445] [Google Scholar]
Dhaked 2016 {published data only}
- Dhaked RD, Meena RS, Maheshwari A, Agarwal US, Purohit S. A randomized comparative trial of two low‐dose oral isotretinoin regimens in moderate to severe acne vulgaris. Indian Dermatology Online Jounal 2016;7(5):378‐85. [PUBMED: 27730033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhir 2008 {published data only (unpublished sought but not used)}
- Dhir R, Gehi NP, Agarwal R, More Y. Oral isotretinoin is as effective as a combination of oral isotretinoin and topical anti‐acne agents in nodulocystic acne. Indian Journal of Dermatology, Venereology and Leprology 2008;74(2):187. [CENTRAL: CN‐01093460] [DOI] [PubMed] [Google Scholar]
Faghihi 2014 {published data only (unpublished sought but not used)}
- Faghihi G, Rakhsshanpour M, Abtahi‐Naeini B, Nilforoushzadeh MA. The efficacy of 5% dapsone gel plus oral isotretinoin versus oral isotretinoin alone in acne vulgaris: a randomized double‐blind study. Advanced Biomedical Research 2014;3:177. [PUBMED: 25250291] [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT2014011015509N1. Comparison of 5% dapsone gel plus oral isotretinoin with oral isotretinoin alone in young patients with moderate to severe facial acne. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014011015509N1 (first received 7 April 2014).
Farrell 1980 {published data only (unpublished sought but not used)}
- Farrell LN, Strauss JS, Stranieri AM. The treatment of severe cystic acne with 13‐cis‐retinoic acid. Evaluation of sebum production and the clinical response in a multiple‐dose trial. Journal of the American Academy of Dermatology 1980;3(6):602‐11. [CENTRAL: CN‐00333819] [DOI] [PubMed] [Google Scholar]
Goldstein 1982 {published data only (unpublished sought but not used)}
- Goldstein JA, Pochi PE, Szott AS, Shalita AR, Thomsen RJ, Strauss JS. Comparative effect of isotretinoin and etretinate on sebaceous gland secretion and acne. Journal of Investigative Dermatology 1981;76(4):327‐8. [CENTRAL: CN‐00321299] [Google Scholar]
- Goldstein JA, Socha‐Szott A, Thomsen RJ, Pochi PE, Shalita AR, Strauss JS. Comparative effect of isotretinoin and etretinate on acne and sebaceous gland secretion. Journal of the American Academy of Dermatology 1982;6 Suppl 4(Pt 2):760‐5. [CENTRAL: CN‐00365999] [DOI] [PubMed] [Google Scholar]
Gollnick 2001 {published data only (unpublished sought but not used)}
- Gollnick HP, Graupe K, Zaumseil RP. Comparison of combined azelaic acid cream plus oral minocycline with oral isotretinoin in severe acne. European Journal of Dermatology 2001;11(6):538‐44. [CENTRAL: CN‐00375280] [PubMed] [Google Scholar]
Jones 1983a {published data only}
- Jones DH, King K, Miller AJ, Cunliffe WJ. A dose‐response study of I3‐cis‐retinoic acid in acne vulgaris. British Journal of Dermatology 1983;108(3):333‐43. [CENTRAL: CN‐00030477] [DOI] [PubMed] [Google Scholar]
Jones 1983b {published data only}
- Jones DH, Forster RA, Mitchell J, Cunliffe WJ. A comparison of 13‐cis‐retinoic acid and erythromycin treatment in severe acne. British Journal of Dermatology 1983;109(Suppl 24):27‐8. [CENTRAL: CN‐00401430] [Google Scholar]
Kapadia 2005 {published data only (unpublished sought but not used)}
- Kapadia NF, Khalid G, Burhany T, Nakhoda T. Comparative efficacy and safety and efficacy of systemic 13‐cis retinoic acid 20mg/day vs. 40mg/day in acne vulgaris. Journal of Pakistan Association of Dermatologists 2005;15(3):238‐41. [CENTRAL: CN‐01632170; EMBASE: 41820790] [Google Scholar]
King 1982 {published data only}
- King K, Jones DH, Daltrey D, Cunliffe WJ. The effect of 13‐cis retinoic acid on the skin microflora of patients with severe acne. Journal of Investigative Dermatology 1982;78(4):328. [CENTRAL: CN‐00321308] [Google Scholar]
- King K, Jones DH, Daltrey DC, Cunliffe WJ. A double‐blind study of the effects of 13‐cis‐retinoic acid on acne, sebum excretion rate and microbial population. British Journal of Dermatology 1982;107(5):583‐90. [CENTRAL: CN‐00568857] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only (unpublished sought but not used)}
- Lee JW, Kim HK, Park MK, Yoo KH, Park KY, Li K, et al. Effectiveness of conventional, low‐dose and intermittent oral isotretinoin in the treatment of acne: a randomized, controlled comparative study. Journal of Dermatology 2010;37(Suppl 1):132. [EMBASE: 70284853] [DOI] [PubMed] [Google Scholar]
- Lee JW, Yoo KH, Park KY, Han TY, Li K, Seo SJ, et al. Effectiveness of conventional, low‐dose and intermittent oral isotretinoin in the treatment of acne: a randomized, controlled comparative study. British Journal of Dermatology 2011;164(6):1369‐75. [CENTRAL: CN‐00813000] [DOI] [PubMed] [Google Scholar]
Leheta 2011 {published data only}
- Leheta T, Garem Y, Abdel Hay R. Treatment of mild to moderate acne with three different modalities. British Journal of Dermatology 2011;165(Suppl 1):98. [CENTRAL: CN‐00843838] [Google Scholar]
Lester 1985 {published data only}
- Lester RS, Schachter GD, Light MJ. Isotretinoin and tetracycline in the management of severe nodulocystic acne. International Journal of Dermatology 1985;24(4):252‐7. [CENTRAL: CN‐00038541] [DOI] [PubMed] [Google Scholar]
Oprica 2007 {published data only (unpublished sought but not used)}
- Oprica C, Emtestam L, Hagstromer L, Nord CE. Clinical and microbiological comparisons of isotretinoin vs. tetracycline in acne vulgaris. Acta Dermato‐Venereologica 2007;87(3):246‐54. [CENTRAL: CN‐00589293] [DOI] [PubMed] [Google Scholar]
Peck 1982 {published data only}
- Peck GL, Olsen TG, Butkus D, Pandya M, Arnaud‐Battandier J, Gross EG, et al. Isotretinoin versus placebo in the treatment of cystic acne. A randomized double‐blind study. Journal of the American Academy of Dermatology 1982;6(4 Pt 2 Suppl):735‐45. [CENTRAL: CN‐00027541] [DOI] [PubMed] [Google Scholar]
Pigatto 1986 {published data only (unpublished sought but not used)}
- Pigatto PD, Finzi AF, Altomare GF, Polenghi MM, Vergani C, Vigotti G. Isotretinoin versus minocycline in cystic acne: a study of lipid metabolism. Dermatologica 1986;172(3):154‐9. [CENTRAL: CN‐00303677] [DOI] [PubMed] [Google Scholar]
Prendiville 1988 {published data only (unpublished sought but not used)}
- Prendiville JS, Logan RA, Russell Jones R. A comparison of dapsone with 13‐cis retinoic acid in the treatment of nodular cystic acne. Clinical and Experimental Dermatology 1988;13(2):67‐71. [CENTRAL: CN‐00180614] [DOI] [PubMed] [Google Scholar]
Rademaker 2013b {published and unpublished data}
- ACTRN12612000062820. Randomized, double‐blind, placebo‐controlled, parallel group clinical study of Isotretinoin 5 mg capsules (once daily) in the treatment of persistent low grade adult acne for 16 weeks followed by an open‐label phase of 16 weeks ‐ Protocol Number: ZPS‐ 365. www.anzctr.org.au/ACTRN12612000062820.aspx (first received 12 January 2012).
- Cooney P. Isotretinoin ongoing trial [personal communication]. E mail to: C Costa 12 February 2013.
- Rademaker M, Birchall N, Wishart J. Isotretinoin 5 mg/day for persistent adult acne. Australasian Journal of Dermatology 2013;54(Suppl 2):20. [CENTRAL: CN‐01027222] [Google Scholar]
- Rademaker M, Birchall N, Wishart J. Isotretinoin 5mg/day in the treatment of persistent low grade adult acne. Therapy. 21st Congress of the European Academy of Dermatology and Venereology; 2012 Sep 27‐30; Prague. Prague: European Academy of Dermatology and Venereology, 2012. [DOI] [PubMed]
- Rademaker M, Wishart JM, Birchall NM. Isotretinoin 5 mg daily for low‐grade adult acne vulgaris ‐ a placebo‐controlled, randomized double‐blind study. Journal of the European Academy of Dermatology and Venereology 2013;28(6):747‐54. [CENTRAL: CN‐00993404] [DOI] [PubMed] [Google Scholar]
Shetti 2013 {published data only}
- Shetti SA, Nagesh HN, Hanumantharaya N. A randomized, open‐label, comparative study of efficacy of low‐dose continuous versus low‐dose intermittent oral isotretinoin therapy in moderate‐to‐severe acne vulgaris. National Journal of Physiology, Pharmacy and Pharmacology 2017;7(9):941‐6. [CENTRAL: CN‐01417962] [Google Scholar]
- Shetti SKA, Nagabushan H, Harish MR. A study of low dose continuous versus low dose intermittent oral isotretinoin therapy in moderate to severe acne vulgaris. Indian Journal of Pharmacology 2013;45(Suppl 1):S80. [EMBASE: 71394541] [Google Scholar]
Strauss 1984 {published data only (unpublished sought but not used)}
- Rapini RR, Konecky EA, Schillinger B, Comite H, Exner JH, Strauss JS, et al. Effect of varying dosages of isotretinoin on nodulocystic acne. Journal of Investigative Dermatology 1983;80(4):358. [CENTRAL: CN‐00321214] [Google Scholar]
- Strauss JS, Rapini RP, Shalita AR, Konecky E, Pochi PE, Comite H, et al. Isotretinoin therapy for acne: results of a multicenter dose‐response study. Journal of the American Academy of Dermatology 1984;10(3):490‐6. [CENTRAL: CN‐00334209] [DOI] [PubMed] [Google Scholar]
Strauss 2001 {published data only (unpublished sought but not used)}
- Strauss JS, Leyden JJ, Lucky AW, Lookingbill DP, Drake LA, Hanifin JM, et al. A randomized trial of the efficacy of a new micronized formulation versus a standard formulation of isotretinoin in patients with severe recalcitrant nodular acne. Journal of the American Academy of Dermatology 2001;45(2):187‐95. [CENTRAL: CN‐00349552] [DOI] [PubMed] [Google Scholar]
- Strauss JS, Leyden JJ, Lucky AW, Lookingbill DP, Drake LA, Hanifin JM, et al. Safety of a new micronized formulation of isotretinoin in patients with severe recalcitrant nodular acne: a randomized trial comparing micronized isotretinoin with standard isotretinoin. Journal of the American Academy of Dermatology 2001;45(2):196‐207. [CENTRAL: CN‐00349553] [DOI] [PubMed] [Google Scholar]
Tan 2014 {published data only (unpublished sought but not used)}
- Tan J, Humphrey S, Lynde C, Vender R, Kerrouche N, Audibert F. Topical fixed‐dose adapalene/benzoyl peroxide plus oral doxycycline: an evidence‐based alternative to oral Isotretinoin in the treatment of severe nodular acne. Australasian Journal of Dermatology 2014;55:35‐6. [CENTRAL: CN‐01087981; EMBASE: 71820742] [Google Scholar]
- Tan J, Humphrey S, Vender R, Barankin B, Gooderham M, Kerrouche N, et al. A treatment for severe nodular acne: a randomized investigator‐blinded, controlled, noninferiority trial comparing fixed‐dose adapalene/benzolyl peroxide plus doxycycline vs. oral isotretinoin. British Journal of Dermatology 2014; Vol. 171, issue 6:1508‐16. [CENTRAL: CN‐01039302] [DOI] [PubMed]
Van der Meeren 1983 {published data only (unpublished sought but not used)}
- Meeren HL, Schroeff JG, Stijnen T, Duren JA, Dries HA, Voorst Vader PC. Dose‐response relationship in isotretinoin therapy for conglobate acne. Dermatologica 1983;167(6):299‐303. [CENTRAL: CN‐00197666] [DOI] [PubMed] [Google Scholar]
- Schroeff JG, Meeren HLM, Stijnen T, Dries HAC, Duren JA, Voorst Vader PC. The treatment of acne conglobata using 13‐cis‐retinoic acid (isotretinoin) [De behandeling van acne conglobata met 13‐cis vitamine A‐zuur (isotretinoine)]. Nederlands Tijdschrift voor Geneeskunde 1983;127(41):1857‐62. [CENTRAL: CN‐00032666] [PubMed] [Google Scholar]
Wahab 2008 {published and unpublished data}
- Wahab MA, Rahman MH, Monamie NS, Jamaluddin M, Khondker L, Afroz W. Isotretinoin versus weekly pulse dose azithromycin in the treatment of acne ‐ a comparative study. Journal of Pakistan Association of Dermatologists 2008;18(1):9‐14. [CENTRAL: CN‐00789353] [Google Scholar]
Webster 2014 {published data only (unpublished sought but not used)}
- Hoover KB, Miller CG, Galante NC, Langman CB. A double‐blind, randomized, phase III, multicenter study in 358 pediatric subjects receiving isotretinoin therapy demonstrates no effect on pediatric bone mineral density. Osteoporosis International 2015;26(10):2441‐7. [CENTRAL: CN‐01258376] [DOI] [PubMed] [Google Scholar]
- NCT00975143. A double‐blind, randomized, phase III, parallel group study evaluating the efficacy and safety of CIP‐Isotretinoin in patients with severe recalcitrant nodular acne. clinicaltrials.gov/show/NCT00975143 (first received 11 September 2009).
- Webster GF, Leyden JJ, Gross JA. Results of a phase III, double‐blind, randomized, parallel‐group, non‐inferiority study evaluating the safety and efficacy of isotretinoin‐Lidose in patients with severe recalcitrant nodular acne. Journal of Drugs in Dermatology 2014;13(6):665‐70. [CENTRAL: CN‐00995064; PUBMED: 24918555] [PubMed] [Google Scholar]
References to studies excluded from this review
Herane 2009 {published data only}
- Herane MI, Fuenzalida H, Zegpi E, Pablo C, Espadas MJ, Trullás C, et al. Specific gel‐cream as adjuvant to oral isotretinoin improved hydration and prevented TEWL increase ‐ a double‐blind, randomized, placebo‐controlled study. Journal of Cosmetic Dermatology 2009;8(3):181‐5. [CENTRAL: CN‐00728527] [DOI] [PubMed] [Google Scholar]
Kus 2005 {published data only}
- Kus S, Gun D, Demirçay Z. Vitamin E does not reduce the side‐effects of isotretinoin in the treatment of acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl 1):126. [CENTRAL: CN‐00416083] [DOI] [PubMed] [Google Scholar]
- Kus S, Gun D, Demirçay Z, Sur H. Vitamin E does not reduce the side‐effects of isotretinoin in the treatment of acne vulgaris. International Journal of Dermatology 2005;44(3):248‐51. [CENTRAL: CN‐00515160; EMBASE: 2005143042] [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li B, Geng L, Xu WB, Zhang M, Zhou M. Effects of Qingfei Liangxue Fa on sebum excretion rate and free fatty acid of patients with acne vulgaris. Zhongguo Linchuang Kangfu [Chinese Journal of Clinical Rehabilitation] 2004;8(20):4056‐7. [CENTRAL: CN‐00508753] [Google Scholar]
Lin 1999 {published data only}
- Lin J, Shih I, Yu C. Hemodialysis‐related nodulocystic acne treated with isotretinoin. Nephron 1999;81(2):146‐50. [CENTRAL: CN‐00159587] [DOI] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu CZ, Lei B, Zheng JF. Randomized control study on the treatment of 26 cases of acne conglobata with encircling acupuncture combined with venesection and cupping. Zhen Ci Yan Jiu [Acupuncture Research] 2008;33(6):406‐8. [CENTRAL: CN‐00681580] [PubMed] [Google Scholar]
Shen 2000 {published data only}
- Shen D, Xu X. A randomised controlled trial of compound shecao decoction versus Isotretinoin in the treatment of acne vulgaris. Journal of Clinical Dermatology 2000;29(4):201‐3. [CENTRAL: CN‐00454654] [Google Scholar]
Strauss 2000 {published data only}
- Strauss JS, Gottlieb AB, Jones T, Koo JY, Leyden JJ, Lucky A, et al. Concomitant administration of vitamin E does not change the side effects of isotretinoin as used in acne vulgaris: a randomized trial. Journal of the American Academy of Dermatology 2000;43(5 Pt 1):777‐84. [CENTRAL: CN‐00328297] [DOI] [PubMed] [Google Scholar]
Sun 2000 {published data only}
- Sun HX, Yang W. 33 cases of severe acne treated by capsulae isotretinoin (Tai Ersi treatment). Chinese Journal of Leprosy and Skin Diseases 2000;16(4):273. [CENTRAL: CN‐00454697] [Google Scholar]
Williams 1992 {published data only}
- Williams RE, Doherty VR, Perkins W, Aitchison TC, Mackie RM. Staphylococcus aureus and intra‐nasal mupirocin in patients receiving isotretinoin for acne. British Journal of Dermatology 1992;126(4):362‐6. [CENTRAL: CN‐00083552] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Faghihi 2017 {published data only}
- Faghihi G, Mokhtari F, Fard NM, Motamedi N, Hosseini SM. Comparing the efficacy of low dose and conventional dose of oral isotretinoin in treatment of moderate and severe acne vulgaris. Journal of Research in Pharmacy Practice 2017;6(4):233‐8. [PUBMED: 29417084] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
IRCT201104094310N6 {published data only}
- IRCT201104094310N6. Comparison of therapeutic effects of oral erythromycin along with low dose oral isotretinoin and oral erythromycin and low dose flutamide versus doxycyclin in female severe acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201104094310N6 (first received 19 April 2014).
IRCT2013110315246N1 {published data only}
- IRCT2013110315246N1. Acne cure rate in patients hyperandrogenism with two drugs of decuttane and cyproterone compound [Comparison of therapiotic effects of oral flutamide and cyproteron compound on female moderate acne]. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013110315246N1 (first received 14 December 2013).
Additional references
ACORN 2013
- Acne Core Outcomes Research Network (ACORN). sites.psu.edu/acnecoreoutcomes/ (accessed 16 October 2016).
Adityan 2009
- Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian Journal of Dermatology, Venereology and Leprology 2009;75(3):323‐6. [PUBMED: 19439902] [DOI] [PubMed] [Google Scholar]
Aksu 2012
- Aksu AEK, Metintas S, Saracoglu ZN, Gurel G, Sabuncu I, Arikan I, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. Journal of the European Academy of Dermatology and Venereology 2012;26(12):1503‐9. [PUBMED: 22070422] [DOI] [PubMed] [Google Scholar]
Alhusayen 2013
- Alhusayen RO, Juurlink DN, Mamdani MM, Morrow RL, Shear NH, Dormuth CR, et al. Isotretinoin use and the risk of inflammatory bowel disease: a population‐based cohort study. Journal of Investigative Dermatology 2013;133(4):907‐12. [PUBMED: 23096714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amado 2006
- Amado JM, Matos ME, Abreu AM, Loureiro L, Oliveira J, Verde A, et al. The prevalence of acne in the north of Portugal. Journal of the European Academy of Dermatology and Venereology 2006;20(10):1287‐95. [PUBMED: 17062047] [DOI] [PubMed] [Google Scholar]
Arowojolu 2012
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004425.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustin 2011
- Augustin MI, Herberger K, Hintzen S, Heigel H, Franzke N, Schäfer I. Prevalence of skin lesions and need for treatment in a cohort of 90,880 workers. British Journal of Dermatology 2011;165(4):865‐73. [PUBMED: 21623753] [DOI] [PubMed] [Google Scholar]
Azoulay 2008
- Azoulay L, Blais L, Koren G, LeLorier J, Berard A. Isotretinoin and the risk of depression in patients with acne vulgaris: a case‐crossover study. Journal of Clinical Psychiatry 2008;69(4):526‐32. [PUBMED: 18363422] [DOI] [PubMed] [Google Scholar]
Ballanger 2006
- Ballanger F, Baudry P, N'Guyen JM, Khammari A, Dréno B. Heredity: a prognostic factor for acne. Dermatology 2006;212(2):145‐9. [PUBMED: 16484821] [DOI] [PubMed] [Google Scholar]
Barbaric 2016
- Barbaric J, Abbott R, Posadzki P, Car M, Gunn LH, Layton AM, et al. Light therapies for acne. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD007917.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bech 2001
- Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. Journal of Affective Disorders 2001;66(2‐3):159‐64. [PUBMED: 11578668] [DOI] [PubMed] [Google Scholar]
Bernstein 2009
- Bernstein CN, Nugent Z, Longobardi T, Blanchard JF. Isotretinoin is not associated with inflammatory bowel disease: a population‐based case–control study. American Journal of Gastroenterology 2009;104(11):2774‐8. [PUBMED: 19623167] [DOI] [PubMed] [Google Scholar]
Binder 2004
- Binder DK, Horton JC, Lawton MT, McDermott MW. Idiopathic intracranial hypertension. Neurosurgery 2004;54(3):538‐51. [PUBMED: 15028127] [DOI] [PubMed] [Google Scholar]
Brokelman 2012
- Brokelman RB, Haverkamp D, Loon C, Hol A, Kampen A, Veth R. The validation of the visual analogue scale for patient satisfaction after total hip arthroplasty. European Orthopaedics and Traumatology 2012;3(2):101‐5. [PUBMED: 22798966] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 1984
- Burke BM, Cunliffe WJ. The assessment of acne vulgaris – the Leeds technique. British Jounal of Dermatology 1984;111(1):83‐92. [PUBMED: 6234917] [DOI] [PubMed] [Google Scholar]
Burton 1971
- Burton JL, Cunliffe WJ, Stafford I, Shuster S. The prevalence of acne vulgaris in adolescence. British Journal of Dermatology 1971;85(2):119‐26. [PUBMED: 4255129] [DOI] [PubMed] [Google Scholar]
Bérard 2007
- Bérard A, Azoulay L, Koren G, Blais L, Perreault S, Oraichi D. Isotretinoin, pregnancies, abortions and birth defects: a population‐based perspective. British Journal of Clinical Pharmacology 2007;63(2):196‐205. [PUBMED: 17214828] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2015
- Cao H, Yang G, Wang Y, Liu JP, Smith CA, Luo H, et al. Complementary therapies for acne vulgaris. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD009436.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2010
- Cheng CE, Irwin B, Mauriello D, Liang L, Pappert A, Kimball AB. Self‐reported acne severity, treatment, and belief patterns across multiple racial and ethnic groups in adolescent students. Pediatric Dermatology 2010;27(5):446‐52. [PUBMED: 20796234] [DOI] [PubMed] [Google Scholar]
Chia 2005
- Chia CY, Lane W, Chibnall J, Allen A, Siegfried E. Isotretinoin therapy and mood changes in adolescents with moderate to severe acne: a cohort study. Archives of Dermatology 2005;141(5):557‐60. [PUBMED: 15897376 ] [DOI] [PubMed] [Google Scholar]
Clark 1995
- Clark SM, Cunliffe WJ. Acne flare and isotretinoin ‐ incidence and treatment. British Journal of Dermatology 1995;133(Suppl 45):26. [Google Scholar]
Coates 1997
- Coates P, Adams CA, Cunliffe WJ, McGinley KT, Eady EA, Leyden JJ, et al. Does oral isotretinoin prevent propionibacterium acnes resistance?. Dermatology 1997;195(Suppl 1):4‐9. [PUBMED: 9310739] [DOI] [PubMed] [Google Scholar]
Cohen 2007
- Cohen J, Adams S, Patten S. No association found between patients receiving isotretinoin for acne and the development of depression in a Canadian prospective cohort. Canadian Journal of Clinical Pharmacology 2007;14(2):e227‐33. [PUBMED: 17556790] [PubMed] [Google Scholar]
Colburn 1983
- Colburn WA, Gibson DM, Wiens RE, Hanigan JJ. Food increases the bioavailability of isotretinoin. Journal of Clinical Pharmacology 1983;23(11‐12):534‐9. [PUBMED: 6582073] [DOI] [PubMed] [Google Scholar]
Collier 2008
- Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. Journal of the American Academy of Dermatology 2008;58(1):56‐9. [PUBMED: 17945383] [DOI] [PubMed] [Google Scholar]
Crockett 2010
- Crockett SD, Porter CQ, Martin CF, Sandler RS, Kappelman MD. Isotretinoin use and the risk of inflammatory bowel disease: a case–control study. American Journal of Gastroenterology 2010;105(9):1986‐93. [PUBMED: 20354506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunliffe 1969
- Cunliffe WJ, Shuster S. The rate of sebum excretion in man. British Journal of Dermatology 1969;81(9):697‐704. [PUBMED: 4243403] [DOI] [PubMed] [Google Scholar]
Cunliffe 1975
- Cunliffe WJ, Williams SM, Tan SG. Sebum excretion rate investigations. A new absorbent paper. British Journal of Dermatology 1975;93(3):347. [PUBMED: 1191540] [DOI] [PubMed] [Google Scholar]
Cunliffe 1981
- Cunliffe WJ. Acne. Update Postgraduate Centre Series. Petersfield: Update Publications Ltd, 1981. [Google Scholar]
Cunliffe 1997
- Cunliffe WJ, Kerkhof PC, Caputo R, Cavicchini S, Cooper A, Fyrand OL, et al. Roaccutane treatment guidelines: results of an international survey. Dermatology 1997;194(4):351‐7. [PUBMED: 9252756] [DOI] [PubMed] [Google Scholar]
Cunliffe 2003
- Cunliffe WJ, Meynadier J, Alirezai M, George SA, Coutts I, Roseeuw DI, et al. Is combined oral and topical therapy better than oral therapy alone in patients with moderate to moderately severe acne vulgaris? A comparison of the efficacy and safety of lymecycline plus adapalene gel 0.1%, versus lymecycline plus gel vehicle. Journal of the American Academy of Dermatology 2003;49(3 Suppl):S218‐26. [DOI] [PubMed] [Google Scholar]
Dai 1992
- Dai WS, LaBraico JM, Stern RS. Epidemiology of isotretinoin exposure during pregnancy. Journal of the American Academy of Dermatology 1992;26(4):509‐606. [PUBMED: 1597546] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [DOI] [PubMed] [Google Scholar]
Del Rosso 2012
- Rosso JQ. Face to face with oral isotretinoin: a closer look at the spectrum of therapeutic outcomes and why some patients need repeated courses. Journal of Clinical and Aesthetic Dermatology 2012;5(11):17‐24. [PUBMED: 23198008] [PMC free article] [PubMed] [Google Scholar]
Demircay 2008
- Demircay Z, Kus S, Sur H. Predictive factors for acne flare during isotretinoin treatment. European Journal of Dermatology 2008;18(4):452‐6. [PUBMED: 18573721] [DOI] [PubMed] [Google Scholar]
Dispenza 2012
- Dispenza MC, Wolpert EB, Gilliland KL, Dai P, Cong Z, Nelson AM, Thiboutot DM. Systemic isotretinoin therapy normalizes exaggerated TLR‐2‐ mediated innate immune responses in acne patients. Journal of Investigative Dermatology 2012;132(9):2198‐205. [PUBMED: 22513780] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doshi 1997
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. International Journal of Dermatology 1997;36(6):416‐8. [PUBMED: 9248884] [DOI] [PubMed] [Google Scholar]
Downing 1968
- Downing DT. Photodensitometry in the thin‐layer chromatographic analysis of neutral lipids. Journal of Chromatography 1968;38(1):91‐9. [PUBMED: 5688076] [DOI] [PubMed] [Google Scholar]
DUETs 2007
- UK Database of Uncertainties about the Effects of Treatments (DUETs). What are the long‐term effects of taking isotretinoin for acne? In particular, if it raises cholesterol so significantly during the course of treatment, is there any evidence to show future problems linked to this?. www.library.nhs.uk/duets/ViewResource.aspx?resID=302864&tabID=294 (accessed 23 March 2014).
DUETs 2009
- UK Database of Uncertainties about the Effects of Treatments (DUETs). Early intervention with oral isotretinoin versus alternative treatments for moderate or mild acne. www.library.nhs.uk/duets/ViewResource.aspx?resID=321332&tabID=296 (accessed 23 March 2014).
DUETs 2010
- UK Database of Uncertainties about the Effects of Treatments (DUETs). Is there a causative association between oral isotretinoin and depression and suicidal behaviour in acne patients?. www.library.nhs.uk/duets/ViewResource.aspx?resID=321407&tabID=297 (accessed 23 March 2014).
DUETs 2011
- UK Database of Uncertainties about the Effects of Treatments (DUETs). Low dose versus standard dose isotretinoin for acne. www.library.nhs.uk/duets/ViewResource.aspx?resID=407423&tabID=297 (accessed 23 March 2014).
Engels 2000
- Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta‐analysis: an empirical study of 125 meta‐analyses. Statistics in Medicine 2000;19(13):1707‐28. [DOI] [PubMed] [Google Scholar]
Etminan 2013
- Etminan M, Bird ST, Delaney JA, Bressler B, Brophy JM. Isotretinoin and risk for inflammatory bowel disease: a nested case‐control study and meta‐analysis of published and unpublished data. JAMA Dermatology 2013;149(2):216‐20. [PUBMED: 23426479] [DOI] [PubMed] [Google Scholar]
Falcon 1986
- Falcon RH, Lee WL, Shalita AR, Suntharalingam K, Fikrig SM. In vitro effect of isotretinoin on monocyte chemotaxis. Journal of Investigative Dermatology 1986;86(5):550‐2. [PUBMED: 3462263] [DOI] [PubMed] [Google Scholar]
Ferahbas 2004
- Ferahbas A, Turan MT, Esel E, Utas S, Kutlugun C, Kilic CG. A pilot study evaluating anxiety and depressive scores in acne patients treated with isotretinoin. Journal of Dermatological Treatment 2004;15(3):153‐7. [PUBMED: 15204147] [DOI] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology life quality index (DLQI) ‐ a simple practical measure for routine clinical use. Clinical and Experimental Dermatology 1994;19(3):210‐6. [PUBMED: 8033378] [DOI] [PubMed] [Google Scholar]
Garner 2012
- Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD002086.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghodsi 2009
- Ghodsi ZS, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: a community‐based study. Journal of the European Academy of Dermatology and Venereology 2009;129(9):2136‐41. [PUBMED: 19282841] [DOI] [PubMed] [Google Scholar]
Gollnick 2003
- Gollnick H. Current concepts of the pathogenesis of acne: implications for drug treatment. Drugs 2003;63(15):1579‐96. [PUBMED: 12887264] [DOI] [PubMed] [Google Scholar]
Gollnick 2016
- Gollnick HP, Bettoli V, Lambert J, Araviiskaia E, Binic I, Dessinioti C, et al. A consensus‐based practical and daily guide for the treatment of acne patients. Journal of the European Academy of Dermatology and Venereology 2016;30(9):1480‐90. [PUBMED: 27177989] [DOI] [PubMed] [Google Scholar]
Goulden 1999
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. Journal of the American Academy of Dermatology 1999;41(4):577‐80. [PUBMED: 10495379] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to 1 November 2018). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] [DOI] [PubMed] [Google Scholar]
Halvorsen 2011
- Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population‐based study. Journal of Investigative Dermatology 2011;131(2):363‐70. [PUBMED: 20844551] [DOI] [PubMed] [Google Scholar]
Harms 1986
- Harms M, Masouyé I, Radeff B. The relapses of cystic acne after isotretinoin treatment are age‐related: a long‐term follow‐up study. Dermatologica 1986;172(3):148‐53. [PUBMED: 2938992] [DOI] [PubMed] [Google Scholar]
Hazen 1983
- Hazen PG, Carney JF, Walker AE, Stewart JJ. Depression ‐ a side effect of 13‐cis‐retinoic acid therapy. Journal of the American Academy of Dermatology 1983;9(2):278‐9. [PUBMED: 6577039] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557–60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holland 2004
- Holland DB, Jeremy AH, Roberts SG, Seukeran DC, Layton AM, Cunliffe WJ. Inflammation in acne scarring: a comparison of the responses in lesions from patients prone and not prone to scar. British Journal of Dermatology 2004;150(1):72‐81. [PUBMED: 14746619] [DOI] [PubMed] [Google Scholar]
Hull 2000
- Hull PR, Demkiw‐Bartel C. Isotretinoin use in acne: prospective evaluation of adverse events. Journal of Cutaneous Medicine & Surgery 2000;4(2):66‐70. [PUBMED: 11179927] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Jick 2000
- Jick SS, Kremers HM, Vasilakis‐Scaramozza C. Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Archives of Dermatology 2000;136(10):1231‐6. [PUBMED: 11030769] [DOI] [PubMed] [Google Scholar]
Jones 1983
- Jones DH, King K, Miller AJ, Cunliffe WJ. A dose‐response study of I3‐cis‐retinoic acid in acne vulgaris. British Journal of Dermatology 1983;108(3):333‐43. [PUBMED: 6219690] [DOI] [PubMed] [Google Scholar]
Karimkhani 2017
- Karimkhani C, Dellavale RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatology 2017;153(5):406‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katsambas 2014
- Katsambas AD, Cunliffe WJ, Zouboulis CC. Clinical aspects of acne vulgaris. In: Zouboulis CC, Katsambas AD, Kligman AM editor(s). Pathogenesis and Treatment of Acne and Rosacea. 1. Berlin Heidelberg: Springer‐Verlag, 2014:213‐22. [Google Scholar]
Kaymak 2009
- Kaymak Y, Taner E, Taner Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. International Journal of Dermatology 2009;48(1):41‐6. [PUBMED: 19126049] [DOI] [PubMed] [Google Scholar]
Kilkenny 1998
- Kilkenny M, Merlin K, Plunkett A, Marks R. The prevalence of common skin conditions in Australian school students: 3. acne vulgaris. British Journal of Dermatology 1998;139(5):840‐5. [PUBMED: 9892951] [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll‐like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005;211(3):193‐8. [PUBMED: 16205063] [DOI] [PubMed] [Google Scholar]
Kontaxakis 2009
- Kontaxakis VP, Skourides D, Ferentinos P, Havaki‐Kontaxaki BJ, Papadimitriou GN. Isotretinoin and psychopathology: a review. Annals of General Psychiatry 2009;8(2):1‐8. [PUBMED: 19154613] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurokawa 2009
- Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Experimental Dermatology 2009;18(10):821‐32. [PUBMED: 19555434] [DOI] [PubMed] [Google Scholar]
Lammer 1985
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. New England Journal of Medicine 1985;313(14):837‐41. [PUBMED: 3162101] [DOI] [PubMed] [Google Scholar]
Layton 2009
- Layton A. The use of isotretinoin in acne. Dermato‐Endocrinology 2009;1(3):162‐9. [PUBMED: 20436884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Layton 2010
- Layton AM. Disorders of sebaceous glands. In: Burns T, Breathnach S, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. 8th Edition. Vol. 2, Oxford: Blackwell Publishing, 2010:38‐66. [Google Scholar]
Lee 2016
- Lee SY, Jamal MM, Nguyen ET, Bechtold ML, Nguyen DL. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta‐analysis. European Journal of Gastroenterology & Hepatology 2016;28(2):210‐6. [PUBMED: 26545085] [DOI] [PubMed] [Google Scholar]
Lehmann 2001
- Lehmann HP, Andrews JS, Robinson KA, Holloway VL, Goodman SN. Management of acne. www.ncbi.nlm.nih.gov/books/NBK33218/ (accessed prior to 9 November 2018). [PUBMED: 11898669] 11898669
Lehucher‐Ceyrac 1999
- Lehucher‐Ceyrac D, Salmoniere P, Chastang C, Morel P. Predictive factors for failure of isotretinoin treatment in acne patients: results from a cohort of 237 patients. Dermatology 1999;198(3):278‐83. [PUBMED: 10393453] [DOI] [PubMed] [Google Scholar]
Leyden 2014
- Leyden JJ, Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. Journal of Clinical and Aesthetic Dermatology 2014;7(2 Suppl):S3‐21. [PUBMED: 24688620] [PMC free article] [PubMed] [Google Scholar]
Lidén 1980
- Lidén S, Göransson K, Odsell L. Clinical evaluation in acne. Acta Dermato‐Venereologica 1980;Suppl 89:47‐52. [PubMed] [Google Scholar]
Magin 2005
- Magin P, Pond D, Smith W. Isotretinoin, depression and suicide: a review of the evidence. British Journal of General Practice 2005;55(511):134‐8. [PUBMED: 15720936] [PMC free article] [PubMed] [Google Scholar]
Mallon 1999
- Mallon E, Newton JN, Klassen A, Stewart‐Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. British Journal of Dermatology 1999;140(4):672‐6. [PUBMED: 10233319] [DOI] [PubMed] [Google Scholar]
Marqueling 2005
- Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Seminars in Cutaneous Medicine and Surgery 2005;24(2):92‐102. [PUBMED: 16092797] [DOI] [PubMed] [Google Scholar]
McGrath 2010
- McGrath EJ, Lovell CR, Gillison F, Darvay A, Hickey JR, Skevington SM. A prospective trial of the effects of isotretinoin on quality of life and depressive symptoms. British Journal of Dermatology 2010;163(6):1323‐9. [PUBMED: 21137117] [DOI] [PubMed] [Google Scholar]
MedDRA 2016
- International Council for Harmonisation (ICH). MedDra (Medical Dictionary for Regulatory Activities Terminology). www.meddra.org (accessed 16 October 2016).
Miles 1938
- Miles AA, Misra SS. The estimation of the bacteriocidal power of blood. Journal of Hygiene 1938;38(6):732. [PUBMED: 20475467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Min 2013
- Min S, Kong HJ, Yoon C, Kim HC, Suh DH. Development and evaluation of an automatic acne lesion detection program using digital image processing. Skin Research and Technology 2013;19(1):e423‐32. [DOI] [PubMed] [Google Scholar]
Nast 2010
- Nast A, Bayerl C, Borelli C, Degitz K, Dirschka T, Erdmann R, et al. S2k – Guideline on the therapy of acne. Journal of the German Society of Dermatology 2010;379(9813):361‐72. [DOI] [PubMed] [Google Scholar]
Nelson 2009a
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Temporal changes in gene expression in the skin of patients treated with isotretinoin provide insight into its mechanism of action. Dermato‐Endocrinology 2009;1(3):177‐87. [PUBMED: 20436886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2009b
- Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Isotretinoin temporally regulates distinct sets of genes in patient skin. Journal of Investigative Dermatology 2009;129(4):1038‐42. [PUBMED: 18987667] [DOI] [PubMed] [Google Scholar]
Ng 2002
- Ng CH, Tam MM, Celi E, Tate B, Schweitzer I. Prospective study of depressive symptoms and quality of life in acne vulgaris patients treated with isotretinoin compared to antibiotic and topical therapy. Australasian Journal of Dermatology 2002;43(4):262‐8. [PUBMED: 12423432] [DOI] [PubMed] [Google Scholar]
Plewig 2000
- Plewig G, Kligman AM. Acne and Rosacea. 3rd Edition. Berlin Heidelberg: Springer‐Verlag, 2000. [Google Scholar]
Pochi 1991
- Pochi PE, Shalita AR, Strauss JS, Webster SB, Cunliffe WJ, Katz HI, et al. Report of the consensus conference on acne classification. Journal of the American Academy of Dermatology 1991;24(3):495‐500. [PUBMED: 1829466] [DOI] [PubMed] [Google Scholar]
Racine 2014
- Racine A, Cuerq A, Bijon A, Ricordeau P, Weill A, Allemand H, et al. Isotretinoin and risk of inflammatory bowel disease: a French nationwide study. American Journal of Gastroenterology 2014;109(4):563‐9. [PUBMED: 24535094] [DOI] [PubMed] [Google Scholar]
Rademaker 2013a
- Rademaker M. Isotretinoin: dose, duration and relapse. What does 30 years of usage tell us?. Australasian Journal of Dermatology 2013;54(3):157‐62. [PUBMED: 23013115] [DOI] [PubMed] [Google Scholar]
Rashtak 2014
- Rashtak S, Khaleghi S, Pittelkow MR, Larson JJ, Lahr BD, Murray JA. Isotretinoin exposure and risk of inflammatory bowel disease. JAMA Dermatology 2014;150(12):1322‐6. [PUBMED: 25207875] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocha 2014
- Rocha MA, Costa CS, Bagatin E. Acne vulgaris: an inflammatory disease even before the onset of clinical lesions. Inflammation & Allergy Drug Targets 2014;13(3):162‐7. [PUBMED: 24909146] [DOI] [PubMed] [Google Scholar]
SBD 2006
- SBD (Sociedade Brasileira de Dermatologia). Nosologic profile of dermatologic visits in Brazil [Perfil nosológico das consultas dermatológicas no Brasil]. Anais Brasileiro de Dermatologia 2006;81(6):545‐54. [Google Scholar]
Schaefer 2010
- Schaefer C, Meister R, Weber‐Schoendorfer C. Isotretinoin exposure and pregnancy outcome: an observational study of the Berlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy. Archives of Gynecology and Obstetrics 2010;281(2):221‐7. [PUBMED: 19444462] [DOI] [PubMed] [Google Scholar]
Schmitt 2016
- Schmitt J, Deckert S, Alam M, Apfelbacher C, Barbaric J, Bauer A, et al. Report from the kick‐off meeting of the Cochrane Skin Group Core Outcome Set Initiative (CSG‐COUSIN). British Journal of Dermatology 2016;174(2):287‐95. [PUBMED: 26779929] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel groups randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [PUBMED: 20346629] [DOI] [PubMed] [Google Scholar]
Shen 2012
- Shen Y, Wang T, Zhou C, Wang X, Ding X, Tian S, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community‐based study of 17,345 subjects in six cities. Acta Dermato‐Venereologica 2012;92(1):40‐4. [PUBMED: 21710106] [DOI] [PubMed] [Google Scholar]
Sladden 2007
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin. Archives of Dermatology 2007;143(9):1187‐8. [PUBMED: 17875883] [DOI] [PubMed] [Google Scholar]
Stern 2004
- Stern RS. Dermatologists and office‐based care of dermatologic disease in the 21st century. Journal of Investigative Dermatology Symposium Proceedings 2004;9(2):126‐30. [PUBMED: 15083778] [DOI] [PubMed] [Google Scholar]
Strauss 1961
- Strauss JS, Pochi PE. The quantitative gravimetric determination of sebum production. Journal of Investigative Dermatology 1961;36:293‐8. [Google Scholar]
Sundstrom 2010
- Sundstrom A, Alfredsson L, Sjolin‐Forsberg G, Gerden B, Bergman U, Jokinen J. Association of suicide attempts with acne and treatment with isotretinoin: retrospective Swedish cohort study. BMJ 2010;341:c5812. [PUBMED: 21071484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2007
- Tan HH, Tan AW, Barkham T, Yan XY, Zhu M. Community‐based study of acne vulgaris in adolescents in Singapore. British Journal of Dermatology 2007;157(3):547‐51. [PUBMED: 17655737] [DOI] [PubMed] [Google Scholar]
Thiboutot 2009
- Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. Journal of the American Academy of Dermatology 2009;60(5 Suppl):S1‐50. [PUBMED: 19376456 ] [DOI] [PubMed] [Google Scholar]
Turner 2013
- Turner L, Savovic J, Higgins J, Reeves B, Sterne J, et al. Methods Innovation Fund updates: the Cochrane Risk of Bias tool extension for non‐randomized studies and studies of non‐parallel design. Cochrane Methods 2013;Suppl 1:1‐58. [Google Scholar]
Uslu 2008
- Uslu G, Sendur N, Uslu M, Savk E, Karaman G, Eskin M. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. Journal of the European Academy of Dermatology and Venereology 2008;22(4):462‐9. [PUBMED: 18179519] [DOI] [PubMed] [Google Scholar]
Webster 2013
- Webster GF, Leyden JJ, Gross JA. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin‐Lidose) and the innovator isotretinoin formulation: a randomized, 4‐treatment, crossover study. Journal of the American Academy of Dermatology 2013;69(5):762‐7. [PUBMED: 23953888] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379(9813):361‐72. [PUBMED: 21880356] [DOI] [PubMed] [Google Scholar]
Williamson 1965
- Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. Journal of Investigative Dermatology 1965;45(6):498‐503. [PUBMED: 5321315] [DOI] [PubMed] [Google Scholar]
Yazici 2004
- Yazici K, Baz K, Yazici AE, Köktürk A, Tot S, Demirseren D, et al. Disease‐specific quality of life is associated with anxiety and depression in patients with acne. Journal of the European Academy of Dermatology and Venereology 2004;18(4):435‐9. [PUBMED: 15196157] [DOI] [PubMed] [Google Scholar]
Zaenglein 2016
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology 2016;74(5):945‐73. [PUBMED: 26897386] [DOI] [PubMed] [Google Scholar]
Zeichner 2016
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. Journal of Drugs in Dermatology 2016;15(1 Suppl 1):s11‐6. [PUBMED: 26741391] [PubMed] [Google Scholar]
Ziana 2006
- Medicis, the Dermatology Company. Prescribing Information. pi.medicis.us/ziana.pdf (accessed 7 October 2014).
Zomerdijk 2014
- Zomerdijk IM, Ruiter R, Houweling LM, Herings RM, Sturkenbonn MC, Straus SM, et al. Isotretinoin exposure during pregnancy: a population‐based study in The Netherlands. BMJ Open 2014;4(11):e005602. [PUBMED: 25392022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zouboulis 2003
- Zouboulis CC, Piquero‐Martin J. Update and future of systemic acne treatment. Dermatology 2003;206(1):37‐53. [PUBMED: 12566804] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Costa 2011
- Costa CS, Bagatin E, Silva EMK, Lúcio MM, Magin P, Riera R. Oral isotretinoin for acne. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009435] [DOI] [PMC free article] [PubMed] [Google Scholar]


