Abstract
Dementia has become a major health concern for the aging population of the United States. Studies indicate that participation in moderate exercise, with training, has been shown to have a beneficial impact on cognition. Thus, exercise and its effects on cognitive function has become an important area of research. This review summarizes the current literature on the potential mechanisms of the benefits of exercise for cognitive function.
Keywords: cognitive function, dementia, exercise, BDNF, PGC-1α, IL-6
Introduction
Dementia is a major cause of death and disability in the United States(1). Family, friends, and caregivers pay a terrible toll in supporting the individual with dementia(1, 2). Even more concerning, we have no effective therapies for attenuating dementia progression once symptoms commence(1). Thus, the importance of understanding lifestyle factors that can be modified to improve cognitive function cannot be overly stressed. Studies have found that exercise can improve aspects of brain health related to cognition (3–16); however, there are still gaps in knowledge regarding the mechanisms controlling these relationships. This review focuses on exercise-induced influences on cognition and addresses some of the potential mechanisms behind observed improvements in cognitive function with exercise.
Exercise and cognitive function
Exercise has been demonstrated to improve cognitive function in healthy older adults(3–7). A twelve week randomized control trial demonstrated that older adults who participated in the exercise group had improved memory and executive functions when compared to the control group(3). Additionally, an 8 year longitudinal study found that older adults who were physically active during leisure time had better subsequent cognitive function and a slower rate of cognitive decline than those who were not(5). Further, a randomized controlled trial in older adults demonstrated changes in brain activity in specific brain regions with exercise training(11). Other studies, in animals and young adults, support this finding and indicate that exercise can induce changes in the hippocampus(8–10). In mice, running enhanced hippocampal neurogenesis and learning(9). In rats, treadmill running reduced activation of inflammatory signaling pathways in the hippocampus and resulted in better cognitive performance(10). Hippocampal changes are specifically relevant to dementia, as it is important in memory processes and has been of specific focus in the study of Alzheimer’s disease(17) Studies have also shown preservation of both white and gray matter with increased exercise(12–15). A recent study demonstrated that wheel running in mice not only improved cognitive function, but also reduced amyloid β plaques and reduced neuroinflammation associated with aging(16). These studies have demonstrated that exercise is an appropriate modifiable factor for reducing the risk of developing dementia.
Physical activity in youth is also important for cognitive function(8, 15, 18–23). Participation in a physical activity program has been shown to improve executive control and data shows that physical activity may improve childhood cognition(24). Additionally, children participating in an exercise program have improved white matter integrity when compared to children involved in a sedentary after-school program(25). These studies and others have provided the rationale for the application of exercise programs in children and adolescents for cognitive benefits.
Research indicates that different types of exercise can have different effects on the brain. Regular moderate aerobic exercise has been shown to promote antioxidant capacity in brain, while anaerobic or high-intensity exercise, aerobic-exhausted exercise, or the combination of these types of training are believed to reduce antioxidant response(26). Studies have indicated beneficial effects of resistance exercise on cognitive function, potentially by enhancing hippocampal synaptic plasticity-related molecules(27). Studies have shown brain structural changes related to strength training in white matter, gray matter, and putamen volume in the healthy adult brain(14, 28–30). Further, long-term mild, rather than intense exercise and sustained aerobic exercise, rather than high intensity interval or resistance training were found to produce hippocampal neurogenesis(31, 32). Thus, a combination of moderate aerobic and resistance exercise would provide an ideal benefit to overall cognitive function.
Adverse effects of extreme exercise
Although exercise has beneficial effects on brain health, studies indicate that training is important and that acute, extreme, or too vigorous of exercise can be detrimental. Extreme exercise has been shown to lead to an increase in plasma S100B, a proposed marker of blood-brain barrier disruption and brain damage(33). Exercise to exhaustion has resulted in increased brain IL-6 levels in rats, but long-term training protects from an increase in hippocampal IL-6(34). Acute bouts of cardiovascular exercise can momentarily alter executive control and increase performance instability in lower fit individuals, while this was reduced in higher fit individuals(35). Further, a study found that moderate-intensity exercise produced a beneficial effect on cognitive function, but this effect was lost with high-intensity exercise(36). Although many prior studies have documented the beneficial effects of exercise, most studies have not considered the potential adverse effects of extreme exercise. In future studies, researchers should take these adverse effects into consideration. In addition, recommendations about exercise should specify that training is important and that acute and extreme exercise without training may not be beneficial.
Potential mechanisms of improved cognition with exercise
Brain glycogen, a critical energy source for neurons, is primarily localized to astrocytes(37). Prolonged exhaustive exercise with hypoglycemia leads to decreases in brain glycogen(38). However, one study indicates that the brain, like skeletal muscle, overcompensates for the loss of astrocyte glycogen(38). Another study supports the theory that glycogen depletion in astrocytes limits the ability of the brain to accelerate its metabolism during activation(39). This would indicate that regular exercise would increase astrocyte glycogen stores, giving the brain increased protection from future bouts of hypoglycemia and improving cognition.
Many studies in animals have demonstrated that exercise can increase hippocampal neurogenesis or rescue the process from various insults, including: restricted cerebral blood flow, lipopolysaccharide exposure, irradiation, and intracerebroventricular amyloid β injection(29, 31, 32, 36, 40–43). The mechanisms by which this occurs are not completely understood, however research has provided some insights into potential mediators of the process. Studies have implicated brain-derived neurotrophic factor (BDNF), serotonin, and adiponectin in the process of exercise-induced hippocampal neurogenesis(36, 40, 42, 44–48).
Exercise is associated with changes in levels of neurotransmitters, neurotrophic factors, and growth factors, alongside increases in temporal lobe functional connectivity(8, 19, 49–54). One of these factors, BDNF, is indicated to function to alter the brain mitochondrial respiratory efficiency; however the presence of inflammatory cytokines appears to block this function(55). The main function of BDNF in the adult brain is to regulate synapses, with structural and functional effects on both excitatory and inhibitory synapses, in many brain regions(56). BDNF regulates energy homeostasis by controlling patterns of feeding and physical activity, and modulating peripheral glucose metabolism(22, 55–61). The role of BDNF in cognitive impairment is unclear, but does seem to have an important role, as mice with knockout of BDNF in restricted areas of the brain manifest object recognition deficiency(62). Previous studies have shown that mice consuming a high-fat diet increase BDNF in multiple regions of the brain(57, 63, 64). On the other hand, exercise was shown to reverse memory impairment caused by a high fat diet and elevate BDNF in neurons of the hippocampal CA3 region(22, 57–59, 65, 66). Further, recent evidence suggests that myokines released by exercising muscles affect the expression of BDNF synthesis in the dentate gyrus of the hippocampus(67). These studies suggest that BDNF is a key mediator of the effects of exercise on cognitive function.
Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α appears to have a role in facilitating some of the effects of exercise on brain health. PGC-1α has been found to be part of the mechanism by which exercise induces hippocampal BDNF expression(68). One study indicated that exercise training is a more effective at reducing age-associated inflammation than resveratrol supplementation and that PGC-1α was required for these anti-inflammatory effects(69). Exercise training of skeletal muscle changes kynurenine metabolism and protects from stress-induced depression(70–73). Activation of PGC-1α1 can increase skeletal muscle expression of kynurenine aminotransferases, which facilitate the conversion of kynurenine into kynurenic acid, a metabolite unable to cross the blood-brain barrier(73). Reducing plasma kynurenine protects the brain from stress-induced changes associated with depression; skeletal muscle-specific PGC-1α1 transgenic mice have been found to be resistant to depression induced by chronic mild stress or direct kynurenine administration(73–76). These studies indicate that PGC-1α in both muscle and brain may mediate the effects of exercise on cognitive function.
Myokines play roles in maintaining biological homeostasis, including energy metabolism, angiogenesis, and myogenesis(77, 78). Interleukin (IL)-6, among other myokines, is dependent upon contraction and plasma levels increase during exercise; this indicates that it may serve as an exercise factor, providing a potential mechanism for the association between sedentary behavior and many chronic diseases(8, 34, 78–89). Although IL-6 is often thought of as pro-inflammatory, some evidence indicates that it can have anti-inflammatory effects as well. Skeletal muscle derived IL-6 produced during exercise has been shown to decrease the production and activity of IL-1β and TNF-α(90). Wheel running mice were shown to have measurable training effects and significantly lower hippocampal TNF-α and higher IL-6, IL-1rα, and IL-12 expression in the hippocampus compared to controls(91). One study indicated that an exercise-induced increase in IL-6 within the brain may serve a neuroprotective role(92). Additionally, the release of IL-6 from the brain when exercise is prolonged may serve as a signal of metabolic stress within the brain(39). Findings suggest that the systemic inflammatory response to acute exercise is different in lean compared to overweight and obese subjects, with overweight and obese individuals exhibiting a more pronounced increase in inflammatory markers (93). Fatigue associated with recovery from muscle damage due to eccentric exercise has recently been linked to increases in brain and muscle pro-inflammatory cytokines(94). Thus, exercise, particularly with training, may alter production of IL-6 and other myokines to produce a beneficial effect on inflammatory markers and brain health.
Summary and Conclusions
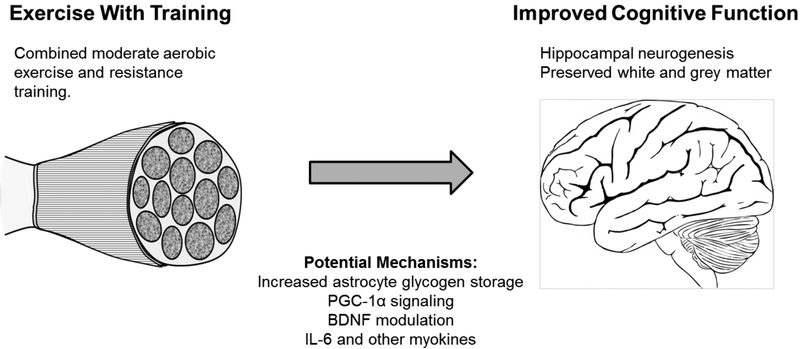
Moderate physical exercise with training appears to be a modifiable lifestyle factor which can provide benefits to cognitive function, these effects and some of the potential mechanisms behind this are summarized in Figure 1. Exercise has been demonstrated to preserve white and gray matter, induce changes in the hippocampus, including neurogenesis, and improve cognitive function. Although the complete details of the mechanisms are not known, researchers have described some aspects of the process. Increased astrocyte glycogen storage, increased expression of BDNF, PGC-1α signaling, and altered skeletal muscle IL-6 production appear to mediate some of the benefits of exercise for cognitive function. Further understanding of these mechanisms may provide insight into potential targets for the development of therapeutics for the prevention and treatment of dementia. Current evidence provides a compelling argument for the participation in moderate physical exercise, consisting of both aerobic and resistance training, as a strategy for improving cognitive function and preventing cognitive decline, perhaps preventing the development of dementia and other neurodegenerative diseases, such as Parkinson’s disease and multiple sclerosis.
Figure 1.
Summary of the effects of exercise on cognitive function and potential mechanisms by which they occur.
Funding
This work was supported by the Richard A. and Nora Eccles Harrison Endowed Chair in Diabetes Research Fund (J.C. Rutledge), and the National Institutes of Health through the following grants: the National Center for Advancing Translational Sciences UL1 TR001860 (L.F. Berglund), National Institute on Aging R01 AG045541 (J.C. Rutledge), National Institute on Arthritis and Musculoskeletal and Skin Diseases R01 AR070031 (S.C. Bodine and J.C. Rutledge), and National Institute of Diabetes and Digestive and Kidney Diseases U24 DK092993–05S1 (J.C. Rutledge) and U24 DK092993 (K. Lloyd). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Alzheimer’s A 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 2.Allen AP, Curran EA, Duggan A, Cryan JF, Chorcorain AN, Dinan TG, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123–64. [DOI] [PubMed] [Google Scholar]
- 3.Nishiguchi S, Yamada M, Tanigawa T, Sekiyama K, Kawagoe T, Suzuki M, et al. A 12-Week Physical and Cognitive Exercise Program Can Improve Cognitive Function and Neural Efficiency in Community-Dwelling Older Adults: A Randomized Controlled Trial. J Am Geriatr Soc. 2015;63(7):1355–63. [DOI] [PubMed] [Google Scholar]
- 4.Chu CH, Chen AG, Hung TM, Wang CC, Chang YK. Exercise and fitness modulate cognitive function in older adults. Psychol Aging. 2015;30(4):842–8. [DOI] [PubMed] [Google Scholar]
- 5.Chu DC, Fox KR, Chen LJ, Ku PW. Components of late-life exercise and cognitive function: an 8-year longitudinal study. Prev Sci. 2015;16(4):568–77. [DOI] [PubMed] [Google Scholar]
- 6.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:12–31. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien J, Ottoboni G, Tessari A, Setti A. One bout of open skill exercise improves cross-modal perception and immediate memory in healthy older adults who habitually exercise. PLoS One. 2017;12(6):e0178739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner G, Herbsleb M, de la Cruz F, Schumann A, Brunner F, Schachtzabel C, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab. 2015;35(10):1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya TK, Pence BD, Ossyra JM, Gibbons TE, Perez S, McCusker RH, et al. Exercise but not (−)-epigallocatechin-3-gallate or beta-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol Behav. 2015;145:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso FDS, Franca EF, Serra FT, Victorino AB, de Almeida AA, Fernandes J, et al. Aerobic exercise reduces hippocampal ERK and p38 activation and improves memory of middle-aged rats. Hippocampus. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Ishii K, Makizako H, Ishiwata K, Oda K, Suzukawa M. Effects of exercise on brain activity during walking in older adults: a randomized controlled trial. J Neuroeng Rehabil. 2017;14(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiology of aging. 2014;35 Suppl 2:S20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Human brain mapping. 2013;34(11):2972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottensteiner M, Leskinen T, Niskanen E, Aaltonen S, Mutikainen S, Wikgren J, et al. Physical activity, fitness, glucose homeostasis, and brain morphology in twins. Med Sci Sports Exerc. 2015;47(3):509–18. [DOI] [PubMed] [Google Scholar]
- 16.He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, et al. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci. 2017;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344(8925):769–72. [DOI] [PubMed] [Google Scholar]
- 18.Khan NA, Hillman CH. The relation of childhood physical activity and aerobic fitness to brain function and cognition: a review. Pediatric exercise science. 2014;26(2):138–46. [DOI] [PubMed] [Google Scholar]
- 19.Labelle V Physical activity may improve measures of cognition in children. Evid Based Med. 2015;20(4):143. [DOI] [PubMed] [Google Scholar]
- 20.Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ. 2015;39(2):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scudder MR, Federmeier KD, Raine LB, Direito A, Boyd JK, Hillman CH. The association between aerobic fitness and language processing in children: implications for academic achievement. Brain and cognition. 2014;87:140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand J Med Sci Sports. 2014;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 23.Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, et al. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134(4):e1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillman CH, Kamijo K, Scudder M. A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Preventive medicine. 2011;52 Suppl 1:S21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krafft CE, Schaeffer DJ, Schwarz NF, Chi L, Weinberger AL, Pierce JE, et al. Improved frontoparietal white matter integrity in overweight children is associated with attendance at an after-school exercise program. Dev Neurosci. 2014;36(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camiletti-Moiron D, Aparicio VA, Aranda P, Radak Z. Does exercise reduce brain oxidative stress? A systematic review. Scand J Med Sci Sports. 2013;23(4):e202–12. [DOI] [PubMed] [Google Scholar]
- 27.Suijo K, Inoue S, Ohya Y, Odagiri Y, Takamiya T, Ishibashi H, et al. Resistance exercise enhances cognitive function in mouse. Int J Sports Med. 2013;34(4):368–75. [DOI] [PubMed] [Google Scholar]
- 28.Rivas DA, Morris EP, Haran PH, Pasha EP, Morais Mda S, Dolnikowski GG, et al. Increased ceramide content and NFkappaB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985). 2012;113(11):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MC, Inoue K, Okamoto M, Liu YF, Matsui T, Yook JS, et al. Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load-free running. Neurosci Lett. 2013;537:6–10. [DOI] [PubMed] [Google Scholar]
- 30.Palmer HS, Haberg AK, Fimland MS, Solstad GM, Moe Iversen V, Hoff J, et al. Structural brain changes after 4 wk of unilateral strength training of the lower limb. J Appl Physiol (1985). 2013;115(2):167–75. [DOI] [PubMed] [Google Scholar]
- 31.Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, et al. Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PLoS One. 2015;10(6):e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594(7):1855–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh SX, Lee JK. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. 2014;44(3):369–85. [DOI] [PubMed] [Google Scholar]
- 34.Aral LA, Pinar L, Goktas G, Deveden EY, Erdogan D. Comparison of hippocampal interleukin-6 immunoreactivity after exhaustive exercise in both exercise-trained and untrained rats. Turkish journal of medical sciences. 2014;44(4):560–8. [DOI] [PubMed] [Google Scholar]
- 35.Labelle V, Bosquet L, Mekary S, Bherer L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain and cognition. 2013;81(1):10–7. [DOI] [PubMed] [Google Scholar]
- 36.Ji JF, Ji SJ, Sun R, Li K, Zhang Y, Zhang LY, et al. Forced running exercise attenuates hippocampal neurogenesis impairment and the neurocognitive deficits induced by whole-brain irradiation via the BDNF-mediated pathway. Biochem Biophys Res Commun. 2014;443(2):646–51. [DOI] [PubMed] [Google Scholar]
- 37.Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89(3):537–52. [DOI] [PubMed] [Google Scholar]
- 38.Matsui T, Ishikawa T, Ito H, Okamoto M, Inoue K, Lee MC, et al. Brain glycogen supercompensation following exhaustive exercise. J Physiol. 2012;590(Pt 3):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol (1985). 2008;104(1):306–14. [DOI] [PubMed] [Google Scholar]
- 40.Littlefield AM, Setti SE, Priester C, Kohman RA. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J Neuroinflammation. 2015;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi DH, Lee KH, Lee J. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep. 2016;13(4):2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DM, Leem YH. Chronic stress-induced memory deficits are reversed by regular exercise via AMPK-mediated BDNF induction. Neuroscience. 2016;324:271–85. [DOI] [PubMed] [Google Scholar]
- 45.Ieraci A, Madaio AI, Mallei A, Lee FS, Popoli M. Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology. 2016;41(13):3070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111(44):15810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kondo M, Nakamura Y, Ishida Y, Shimada S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry. 2015;20(11):1428–37. [DOI] [PubMed] [Google Scholar]
- 48.Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33(19):8270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matta Mello Portugal E, Cevada T, Sobral Monteiro-Junior R, Teixeira Guimaraes T, da Cruz Rubini E, Lattari E, et al. Neuroscience of exercise: from neurobiology mechanisms to mental health. Neuropsychobiology. 2013;68(1):1–14. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Mesa Y, Colie S, Corpas R, Cristofol R, Comellas F, Nebreda AR, et al. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J Gerontol A Biol Sci Med Sci. 2016;71(1):40–9. [DOI] [PubMed] [Google Scholar]
- 51.Zschucke E, Renneberg B, Dimeo F, Wustenberg T, Strohle A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology. 2015;51:414–25. [DOI] [PubMed] [Google Scholar]
- 52.Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clinical interventions in aging. 2014;9:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Deutsches Arzteblatt international. 2011;108(44):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markham A, Bains R, Franklin P, Spedding M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? Br J Pharmacol. 2014;171(8):2206–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–50. [DOI] [PubMed] [Google Scholar]
- 57.Kishi T, Hirooka Y, Nagayama T, Isegawa K, Katsuki M, Takesue K, et al. Calorie restriction improves cognitive decline via up-regulation of brain-derived neurotrophic factor: tropomyosin-related kinase B in hippocampus ofobesity-induced hypertensive rats. Int Heart J. 2015;56(1):110–5. [DOI] [PubMed] [Google Scholar]
- 58.Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgarner KM, Setti S, Diaz C, Littlefield A, Jones A, Kohman RA. Diet-induced obesity attenuates cytokine production following an immune challenge. Behav Brain Res. 2014;267:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10(3):365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kishi T, Sunagawa K. Exercise training plus calorie restriction causes synergistic protection against cognitive decline via up-regulation of BDNF in hippocampus of stroke-prone hypertensive rats. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:6764–7. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obesity. 2013;37(3):382–9. [DOI] [PubMed] [Google Scholar]
- 65.Tsai CL, Chen FC, Pan CY, Wang CH, Huang TH, Chen TC. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology. 2014;41:121–31. [DOI] [PubMed] [Google Scholar]
- 66.Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Frontiers in cellular neuroscience. 2014;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18(5):649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olesen J, Ringholm S, Nielsen MM, Brandt CT, Pedersen JT, Halling JF, et al. Role of PGC-1alpha in exercise training- and resveratrol-induced prevention of age-associated inflammation. Exp Gerontol. 2013;48(11):1274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mudry JM, Alm PS, Erhardt S, Goiny M, Fritz T, Caidahl K, et al. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab Res Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 71.Dieli-Conwright CM, Kiwata JL, Tuzon CT, Spektor TM, Sattler FR, Rice JC, et al. Acute Response of PGC-1alpha and IGF-1 Isoforms to Maximal Eccentric Exercise in Skeletal Muscle of Postmenopausal Women. J Strength Cond Res. 2016;30(4):1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moon HY, van Praag H. Muscle over mind. Cell Metab. 2014;20(4):560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. [DOI] [PubMed] [Google Scholar]
- 74.Godoy JA, Rios JA, Zolezzi JM, Braidy N, Inestrosa NC. Signaling pathway cross talk in Alzheimer’s disease. Cell communication and signaling : CCS. 2014;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18(10):1208–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews Endocrinology. 2012;8(8):457–65. [DOI] [PubMed] [Google Scholar]
- 77.Agergaard J, Reitelseder S, Pedersen TG, Doessing S, Schjerling P, Langberg H, et al. Myogenic, matrix, and growth factor mRNA expression in human skeletal muscle: effect of contraction intensity and feeding. Muscle & nerve. 2013;47(5):748–59. [DOI] [PubMed] [Google Scholar]
- 78.Yoon JH, Kim J, Song P, Lee TG, Suh PG, Ryu SH. Secretomics for skeletal muscle cells: a discovery of novel regulators? Adv Biol Regul. 2012;52(2):340–50. [DOI] [PubMed] [Google Scholar]
- 79.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin--mediators of inflammation and mediators of exercise. Mediators of inflammation. 2013;2013:320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catoire M, Kersten S. The search for exercise factors in humans. FASEB J. 2015;29(5):1615–28. [DOI] [PubMed] [Google Scholar]
- 81.Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:295–303. [DOI] [PubMed] [Google Scholar]
- 82.Capel F, Acquaviva C, Pitois E, Laillet B, Rigaudiere JP, Jouve C, et al. DHA at nutritional doses restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation. J Nutr Biochem. 2015. [DOI] [PubMed] [Google Scholar]
- 83.Lira FS, Rosa Neto JC, Antunes BM, Fernandes RA. The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr Diabetes Rev. 2014;10(6):391–6. [DOI] [PubMed] [Google Scholar]
- 84.Rethorst CD, Greer TL, Toups MS, Bernstein I, Carmody TJ, Trivedi MH. IL-1beta and BDNF are associated with improvement in hypersomnia but not insomnia following exercise in major depressive disorder. Transl Psychiatry. 2015;5:e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit Rev Clin Lab Sci. 2015:1–11. [DOI] [PubMed] [Google Scholar]
- 86.Neefkes-Zonneveld CR, Bakkum AJ, Bishop NC, van Tulder MW, Janssen TW. Effect of long-term physical activity and acute exercise on markers of systemic inflammation in persons with chronic spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2015;96(1):30–42. [DOI] [PubMed] [Google Scholar]
- 87.Reihmane D, Dela F. Interleukin-6: possible biological roles during exercise. Eur J Sport Sci. 2014;14(3):242–50. [DOI] [PubMed] [Google Scholar]
- 88.Pal M, Febbraio MA, Whitham M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol. 2014;92(4):331–9. [DOI] [PubMed] [Google Scholar]
- 89.Chen YW, Apostolakis S, Lip GY. Exercise-induced changes in inflammatory processes: Implications for thrombogenesis in cardiovascular disease. Ann Med. 2014;46(7):439–55. [DOI] [PubMed] [Google Scholar]
- 90.Wood LJ, Nail LM, Winters KA. Does muscle-derived interleukin-6 mediate some of the beneficial effects of exercise on cancer treatment-related fatigue? Oncology nursing forum. 2009;36(5):519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pervaiz N, Hoffman-Goetz L. Freewheel training alters mouse hippocampal cytokines. Int J Sports Med. 2011;32(11):889–95. [DOI] [PubMed] [Google Scholar]
- 92.Funk JA, Gohlke J, Kraft AD, McPherson CA, Collins JB, Jean Harry G. Voluntary exercise protects hippocampal neurons from trimethyltin injury: possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. 2011;25(6):1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christiansen T, Bruun JM, Paulsen SK, Olholm J, Overgaard K, Pedersen SB, et al. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. European journal of applied physiology. 2013;113(6):1635–42. [DOI] [PubMed] [Google Scholar]
- 94.Carmichael MD, Davis JM, Murphy EA, Carson JA, Van Rooijen N, Mayer E, et al. Role of brain macrophages on IL-1beta and fatigue following eccentric exercise-induced muscle damage. Brain Behav Immun. 2010;24(4):564–8. [DOI] [PubMed] [Google Scholar]