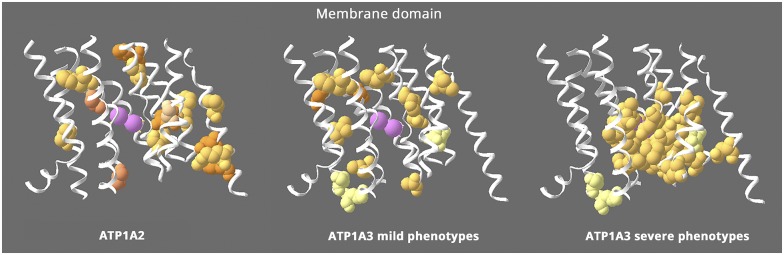
Figure 2. Structure-phenotype relationship in the membrane domain.
This figure shows the transmembrane α-helices, omitting the portions that extend into the extracellular and intracellular spaces. All the ATP1A2 membrane mutations are shown (left), whereas ATP1A3's are divided into 2 groups: milder ATP1A3 phenotypes (middle) with onset from childhood to adult and severe ATP1A3 mutations (right) with onset in infancy. ATP1A2 and the milder ATP1A3 phenotypes have similar distributions that appear to exclude the residues right around the ions (pink = potassium ions). In contrast, the severe ATP1A3 mutations are usually close to the ions. Almost 70% of the severe mutations are in contiguous stretches in M4, M5, and M6 near the ions. In contrast, 100% of the mild mutations are not adjacent to any other mutations, and the few in M4, M5, and M6 are on either side of the contiguous stretches. The distributions highlight 2 unique features: that mild and severe ATP1A3 mutations have different distributions and that ATP1A2 mutations all look like “mild”. In fact, hemiplegic migraine seldom has onset in infancy.17 Equivalent “severe” mutations of ATP1A2 have not been found. Variants of 3 ATP1A3 residues in pale yellow can produce either mild or severe phenotypes. Fourteen ATP1A3 residues altogether have produced both RDP and mild AHC or an intermediate phenotype. Eleven of those were recurrent (with the same or different substitution) or appeared also in ATP1A1 or ATP1A2 patients. Here, 2 had different substitutions in patients with differing phenotypes (S137Y or S137F severe,10 S137del mild53; Q140L severe,10 Q140H mild54), and the third, D923N, reduces affinity for Na+55 and presents as a continuum between AHC and RDP,56,57 severe or mild in the same family.58