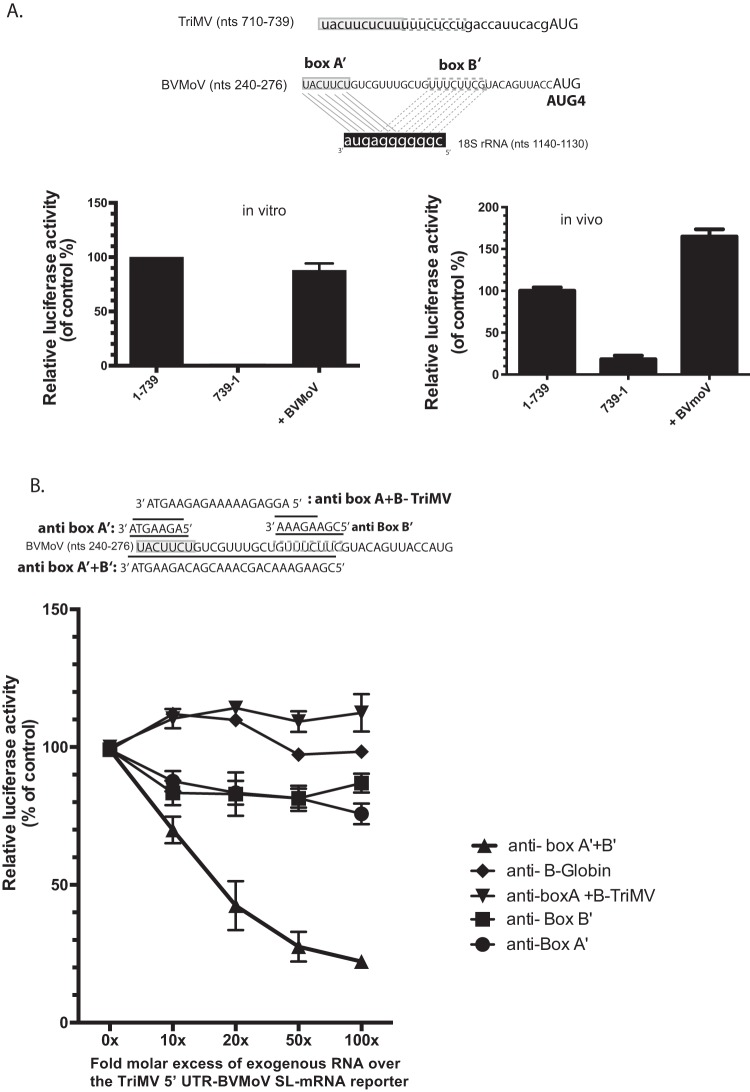
FIG 6.
A 35-nt sequence from the BVMoV leader sequence can functionally substitute for the TriMV YX-AUG function in translation. (A) Boxed are the two uncoupled putative box A′ and box B′ binding sites within the BVMoV 5′ UTR sequence at position nt 240 to 247 and nt 258 to 265 (accession number KY491536.1) upstream of the fourth AUG. The relative luciferase activity of the TriMV 5′ UTR with the last 30 nt swapped with a 35-nt sequence of the BVMoV in wheat germ extract (on the left) and in oat protoplasts (on the right) is relativized to that of the TriMV wild-type sequence in the presence of the strong hairpin. For the oat protoplast assays, the reporter mRNAs were coelectroporated with a m7GpppG-capped polyadenylated renilla mRNA used as an internal control at a 1:10 ratio (B). trans-Inhibition assay of the chimeric TriMV 5′ UTR-BVMoV SL-mRNA construct with increasing molecular excess of antisense single-stranded DNA oligonucleotides targeting the box A′ alone (anti-BoxA′), box B’ (anti-BoxB′) alone, both box A′ and box B′ (anti-BoxB′), and box A and box B of the native TriMV (anti-BoxA+BoxB-TriMV) 18S rRNA binding sites in wheat germ extract. As a control, DNA oligonucleotides targeting unrelated human β-globin sequence were added. A 0- to 100-fold molar excess of the antisense oligonucleotides was added to the reaction.