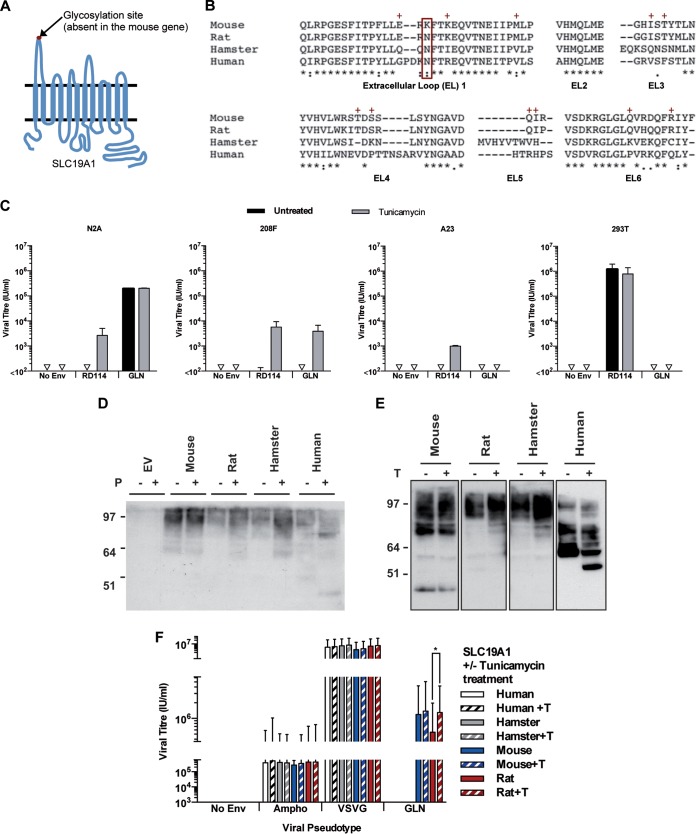
FIG 5.
Effects of glycosylation on GLN tropism. (A) Schematic of SLC9A1 showing the 12 transmembrane domains and the 6 extracellular loops. A putative N-linked glycosylation site is present in the first extracellular loop of all species except for mouse. (B) Clustal analysis of the amino acid sequences of SLC19A1 showing the amino acid similarities of the six extracellular loops between mouse, rat, hamster, and human proteins. The glycosylation site at N56 (boxed in red) is absent in the mouse protein. The red + symbols indicate residues shared by mouse and rat proteins but not human and hamster proteins. (C) Titers of viruses pseudotyped with RD114 or GLN envelopes were measured on different cell lines with or without 16 h of tunicamycin (0.2 μg/ml) pretreatment to remove N-linked glycosylation sites (n = 3; the SD are represented by the error bars). (D) 293T cells were transduced to express HA-tagged SLC19A1 from different species. Cell lysates were treated with PNGase F (shortened to P) to remove N-linked glycans before probing for SLC19A1 by Western blotting using an anti-HA antibody. (E) Cells were treated with tunicamycin (shortened to T) before collection of cell lysates, and SLC19A1 proteins were detected by Western blotting using an anti-HA antibody. (F) The titers of viruses pseudotyped with different Envs were measured on 293T cells expressing HA-tagged SLC19A1 from different species with and without tunicamycin pretreatment.