Borna disease virus (BoDV) is a highly neurotropic RNA virus that belongs to the orthobornavirus genus. A zoonotic orthobornavirus that is genetically related to BoDV has recently been identified in squirrels, thus increasing the importance of understanding the replication and pathogenesis of orthobornaviruses. BoDV replicates in the nucleus and uses alternative mRNA splicing to express viral proteins. However, it is unknown whether the virus uses splicing to create protein isoforms with different functions. The present study demonstrated that the nucleoprotein transcript undergoes splicing and produces four new isoforms in coordination with alternative usage of translation initiation codons. The spliced isoforms showed a distinct intracellular localization, including in the endoplasmic reticulum, and recombinant viruses lacking the splicing signals replicated more efficiently than the wild type. The results provided not only a new regulation of BoDV replication but also insights into how RNA viruses produce protein isoforms from small genomes.
KEYWORDS: bornavirus, Mononegavirales, RNA splicing
ABSTRACT
Targeting of viral proteins to specific subcellular compartments is a fundamental step for viruses to achieve successful replication in infected cells. Borna disease virus 1 (BoDV-1), a nonsegmented, negative-strand RNA virus, uniquely replicates and persists in the cell nucleus. Here, it is demonstrated that BoDV nucleoprotein (N) transcripts undergo mRNA splicing to generate truncated isoforms. In combination with alternative usage of translation initiation sites, the N gene potentially expresses at least six different isoforms, which exhibit diverse intracellular localizations, including the nucleoplasm, cytoplasm, and endoplasmic reticulum (ER), as well as intranuclear viral replication sites. Interestingly, the ER-targeting signal peptide in N is exposed by removing the intron by mRNA splicing. Furthermore, the spliced isoforms inhibit viral polymerase activity. Consistently, recombinant BoDVs lacking the N-splicing signals acquire the ability to replicate faster than wild-type virus in cultured cells, suggesting that N isoforms created by mRNA splicing negatively regulate BoDV replication. These results provided not only the mechanism of how mRNA splicing generates viral proteins that have distinct functions but also a novel strategy for replication control of RNA viruses using isoforms with different subcellular localizations.
IMPORTANCE Borna disease virus (BoDV) is a highly neurotropic RNA virus that belongs to the orthobornavirus genus. A zoonotic orthobornavirus that is genetically related to BoDV has recently been identified in squirrels, thus increasing the importance of understanding the replication and pathogenesis of orthobornaviruses. BoDV replicates in the nucleus and uses alternative mRNA splicing to express viral proteins. However, it is unknown whether the virus uses splicing to create protein isoforms with different functions. The present study demonstrated that the nucleoprotein transcript undergoes splicing and produces four new isoforms in coordination with alternative usage of translation initiation codons. The spliced isoforms showed a distinct intracellular localization, including in the endoplasmic reticulum, and recombinant viruses lacking the splicing signals replicated more efficiently than the wild type. The results provided not only a new regulation of BoDV replication but also insights into how RNA viruses produce protein isoforms from small genomes.
INTRODUCTION
The regulation of the subcellular localization of proteins is critical for them to precisely exert their function and trafficking. Therefore, eukaryotic proteins contain several signal sequences, such as nuclear localization signals (NLSs), nuclear export signal (NESs), Golgi retrieval signals, endoplasmic reticulum (ER) retention signals, and mitochondrion-targeting signals (1–4). In addition to these signal sequences, posttranslational modifications and protein-protein interactions also determine the intracellular distribution of proteins.
Viruses must unerringly control the subcellular localization of their proteins to accomplish replication in host cells. To generate multiple proteins with diverse functions and subcellular localizations from relatively short viral genomes, viruses utilize several transcription and translation mechanisms, such as cotranscriptional editing, mRNA splicing, internal ribosome entry sites (IRESs), ribosomal shunting, leaky scanning, non-AUG initiation, frameshifting, and readthrough (5, 6). For instance, phosphoprotein (P) mRNAs of many Mononegavirales are translated from downstream initiation codons with leaky scanning and ribosomal shunting mechanisms, thereby generating N-terminally truncated protein isoforms with different functions (7). Because organelle-targeting sequences often locate at the N terminus, these P isoforms can localize to distinct subcellular components.
mRNA splicing often deletes sequences encoding organelle-targeting signals in viral proteins, producing isoforms with different subcellular localizations. In human T-cell leukemia virus type 1 (HTLV-1) infection, alternative splicing of the bZIP factor results in different subcellular distributions of its isoforms (8). Furthermore, the L6 region of bovine adenovirus 3 expresses several isoforms with distinct subcellular localizations in infected cells, which may arise by internal initiation of translation and alternative splicing (9). However, the detailed mechanism of how viruses control the subcellular localization of viral proteins by mRNA splicing has not been elucidated.
Borna disease virus 1 (BoDV-1) is a nonsegmented, negative-strand RNA virus that replicates and transcribes in the nucleus. The BoDV genome harbors at least six open reading frames (ORFs), as follows: nucleoprotein (N), X, P, matrix protein (M), glycoprotein (G), and large protein (L) (10). BoDV exploits the regulatory mechanism of the intracellular localization of viral proteins to establish intranuclear persistent infection. The intracellular localization of BoDV ribonucleoproteins (RNPs) is regulated by viral proteins containing NLSs and NESs as well as their interactions. N, P, X, and L harbor NLSs, and N and P contain NESs (11–18). Previous studies have demonstrated that the interaction of accessory protein X with P enhances the nuclear export of P (19). In contrast, P directly binds to N, leading to the retention of N in the nucleus (13, 20). N transcripts encode two isoforms, namely, full-length isoform N (p40) and N-terminally truncated isoform N′ (p38), which is translated from the second initiation codon downstream of the NLS (17). While N mainly localizes to the nucleus, the solo expression of N′ is found in the cytoplasm (12, 13, 17). N′ can modify the nuclear distribution of P via their interaction (13). Furthermore, it has been reported that the expression ratio of N and N′ in cells is important for the elaborate control of BoDV polymerase activity (21, 22). Thus, BoDV strictly regulates the intracellular localization of viral proteins by intrinsic subcellular localization signals and protein-protein interactions to accomplish viral replication and establish persistent infection in the nucleus.
BoDV utilizes the host mRNA splicing machinery for gene expression (23, 24). The ORF of BoDV G overlaps those of M and L in a +1/−2 frame (10). To express M, G, and L, the overlapping protein-coding region produces three different spliced transcripts using the host pre-mRNA splicing machinery. Transcripts lacking introns I and II serve as mRNAs for G and M, respectively (23, 24). mRNAs that lack both introns are for L (23, 24). Furthermore, rare alternative splicing has been observed in the transcripts expressed from the M/G/L overlapping coding region by reverse transcription-PCR (RT-PCR) and Northern blotting (25). These reports have demonstrated that alternative splicing of BoDV mRNAs plays a critical role for viral gene expression. However, no splicing event has been demonstrated in BoDV transcripts, except for the M/G/L polycistronic mRNAs.
The present study comprehensively analyzed the splicing junctions of BoDV transcripts using next-generation sequencing (NGS) and discovered that the N transcript contains two short introns and undergoes splicing. Although full-length N mainly accumulates at intranuclear viral replication sites, called viral speckles of transcripts (vSPOTs), a spliced isoform of N accumulates in the ER. Mutational and structural analyses revealed that intracellular localizations of spliced isoforms of N are regulated by a combined mechanism with alternative initiation sites and splicing of the N transcript. Interestingly, recombinant BoDVs (rBoDVs) lacking splicing signals in N showed the ability to propagate faster than wild-type rBoDV (rBoDV-WT), suggesting that the spliced isoforms of N negatively regulate BoDV replication. These results may provide not only a new strategy for BoDV replication in the nucleus but also new insight into the mechanism of how RNA viruses produce protein isoforms with different subcellular localizations from a single transcript.
RESULTS
RNA sequencing defines the splicing landscape of BoDV transcripts.
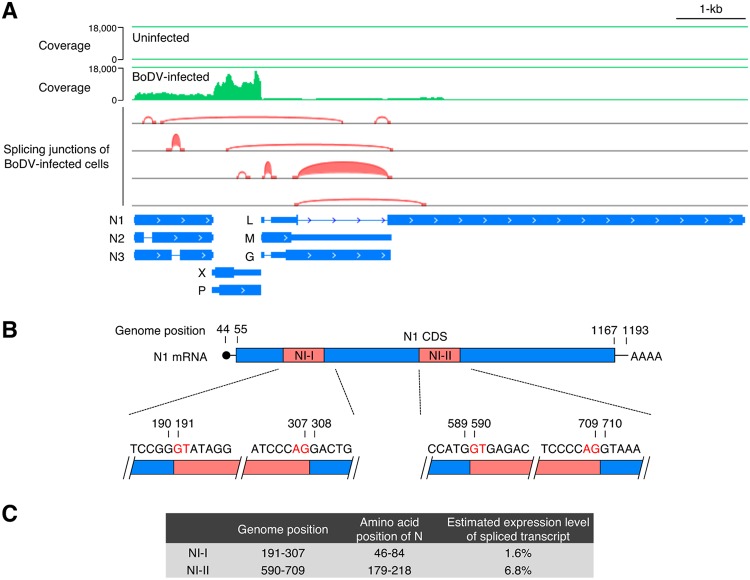
To identify BoDV splicing transcripts, transcriptome analysis of persistently BoDV (strain He/80)-infected and uninfected oligodendroglioma (OL) cells was performed by NGS (Fig. 1A). Sequence reads were mapped to reference genomes of human and BoDV to avoid the mismapping of host mRNAs to the viral genome. In addition to the previously reported splicing patterns in the overlapping M/G/L coding region, two short spliced introns of N, termed NI-I (nucleotides [nt] 191 to 307) and NI-II (nt 590 to 709), were detected within the N sequence in the reads of BoDV-infected OL cells, suggesting that splicing variants of BoDV N mRNA were produced in the infected cells (Fig. 1A). To verify the splicing of N mRNA, RT-PCR was performed using RNAs from BoDV-infected cells. The same splicing variants of N mRNA were observed by NGS (data not shown). Two newly identified splicing variants in the N transcripts harbored canonical eukaryotic splicing signals (GU-AG) and branchpoint sequences (Fig. 1B), strongly suggesting the involvement of host splicing machinery. To estimate the expression levels of the N transcript variants, Mixture of Isoforms (MISO) software (26), which quantitates the expression levels of alternatively spliced genes from RNA sequencing (RNA-seq) data, was used. The levels of splicing of NI-I and NI-II introns were estimated to be 1.6% and 6.8%, respectively (Fig. 1C), indicating that the expression levels of these splicing variants are not high enough to be detected by Northern blotting (10, 27).
FIG 1.
Alternative splicing generates transcript variants of N. (A) IGV browser view showing the coverage and splicing junctions of mapped RNA reads from persistently BoDV-infected OL cells. (B) Map of the splicing donor and acceptor sequences of NI-I and NI-II. (C) Genome positions and amino acid positions corresponding to the N introns and estimated expression levels of spliced N transcripts. Expression levels of spliced N transcripts were estimated from the RNA-seq data of BoDV-infected OL cells using MISO (Mixture of Isoforms) software that quantitates the expression levels of alternatively spliced genes from RNA-seq data.
To elucidate if these splicing events are observed in the acute stage of infection, amplicon deep sequencing of the N transcripts was performed. To obtain the N transcript in the acute stage, OL cells were infected with BoDV, and total RNA was extracted at 4, 8, 16, 24, and 48 h postinfection. As a control, total RNA of persistently BoDV-infected OL and 293T cells was collected. Transcripts of N were amplified by RT-PCR, and the sequences of the amplicon were analyzed by RNA sequencing. Splicing variants of N mRNA were observed in both acute and persistent infection, indicating that the spliced N transcripts are ubiquitously expressed in the course of BoDV infection (Table 1).
TABLE 1.
Numbers of deep sequencing reads containing splice junctions
| Intron | No. of readsa |
||||||
|---|---|---|---|---|---|---|---|
| Acute infection of OL cells |
Persistent infection |
||||||
| 4 hpi | 8 hpi | 16 hpi | 24 hpi | 48 hpi | OL cells | 293T cells | |
| NI-Ib | 665 | 2,224 | 1,913 | 2,142 | 1,948 | 200 | 292 |
| NI-IIb | 6,444 | 8,337 | 4,734 | 3,684 | 6,761 | 3,781 | 3,671 |
| Totalc | 1,755,346 | 2,098,444 | 2,028,286 | 2,015,967 | 2,057,286 | 2,415,536 | 2,068,547 |
hpi, hours postinfection.
The number of reads which harbored the splicing junction of the indicated intron.
The number of reads mapped to the N transcript.
Isoform N3 undergoes posttranslational modification.
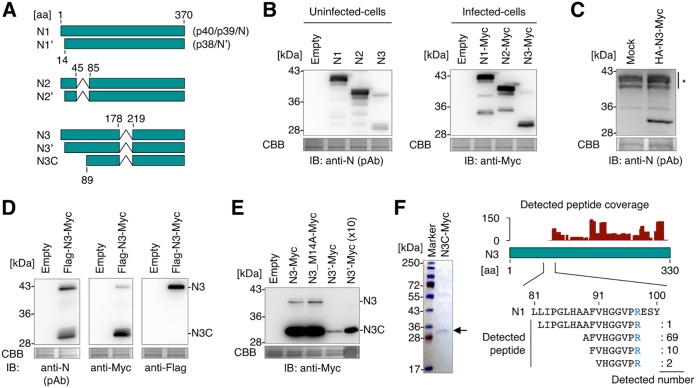
After the splicing of NI-I and NI-II, N transcripts retain the protein-coding sequence in the same reading frame of N, suggesting that these variants produce truncated isoforms of N (Fig. 2A). To further analyze the predicted N isoforms, we designated full-length N and the spliced isoforms, which are translated from the first AUG codon of the N ORF, N1, N2, and N3, and we designated those from the second AUG codon N1′, N2′, and N3′, respectively (Fig. 2A). To investigate the expression pattern of N2 and N3 isoforms, expression plasmids of N2 and N3 were generated. To prevent multiple splicing, silent mutations were introduced at each splicing donor and acceptor site. While N2 was observed at the predicted molecular weight (MW), N3 was observed at a lower position than the expected size where the predicted molecular weight was 36 kDa, in both uninfected and BoDV-infected cells (Fig. 2B). To discriminate the possibility that the transcripts expressed from the plasmid encoding N3 undergo further unexpected splicing events, N3 mRNA was synthesized in vitro and transfected into uninfected cells. As shown in Fig. 2C, transfection of N3 mRNA also produced the shorter isoform, suggesting that this isoform results from posttranslational modification of N3.
FIG 2.
N isoforms expressed from splicing variants of N transcript. (A) Schematic representation of N isoforms. The amino acid positions corresponding to N1 are shown. Synonymous names are shown in parentheses. (B) Detection of N isoforms by Western blotting. Whole-cell lysates of uninfected and persistently BoDV-infected 293T cells transfected with plasmids encoding the indicated N isoforms were used. (C) Detection of N isoforms by Western blotting. Whole-cell lysates of uninfected 293T cells transfected with the mRNA encoding the indicated N isoforms were used. The asterisk represents nonspecific signals. (D) Detection of the N3 short form by Western blotting. The whole-cell lysate of uninfected 293T cells expressing Flag-N3-Myc was used. (E) Detection of N3C-Myc by Western blotting. Whole-cell lysates of uninfected 293T cells expressing the indicated N3 mutants were used. In the ×10 sample, a 10-fold amount of the cell lysate was used for analysis. Coomassie brilliant blue (CBB) staining was used as a loading control. IB, immunoblotting; pAb, polyclonal antibody. (F) Detection of the N terminus of N3C. (Left) Purified N3C-Myc. N3C-Myc was purified from uninfected 293T cells expressing N3C-Myc using an anti-Myc tag antibody. Purified protein was stained with CBB. (Right) Coverage of N3C peptides and the N-terminal fragments of N3C detected by mass spectrometry. The arginine residue of which the peptide bond of the carboxy side is cleaved by trypsin during sample preparation is shown in blue.
To identify the modification of the shorter isoform, Flag-N3-Myc, which has 3×Flag and Myc tags in the N and C termini, respectively, was expressed in uninfected cells. While a 42-kDa band was detected by both anti-Flag and anti-Myc antibodies, the 30-kDa band was detected only by the anti-Myc antibody (Fig. 2D). Because the molecular weight of the full-length product was approximately 40 kDa, the 42-kDa band may represent the full-length Flag-N3-Myc product, and the 30-kDa form may contain the C-terminal fragment of N3, designated N3C here (Fig. 2A). To evaluate if N3′ can be processed into N3C, N3′ was expressed from a plasmid. Although the expression level of N3′ was lower than that of N3, N3′ also clearly expressed N3C (Fig. 2E), indicating an intron NI-II-dependent production of N3C. The N3-encoding plasmid potentially expresses N3′. To discriminate the influence of N3′ on N3C expression from the N3-encoding plasmid, we therefore made a plasmid encoding an N3 mutant, named N3_M14A, which harbors mutations at the second ATG codon in the N3 ORF. As shown in Fig. 2E, N3_M14A also produced N3C, indicating that both N3 and N3′ can be processed into N3C. Note that although the expression level of N3C seems to be high in BoDV-infected cells compared to uninfected cells (Fig. 2B), it resulted from the difference of primary antibodies used in these two panels. In addition, the signals of the shorter isoforms appear to be weaker than those of the full-length proteins when polyclonal anti-N antibodies are used for detection. This is probably due to the difference in the numbers of epitopes that they harbor.
The N3C protein was sequenced to identify the N terminus of this product. To this end, N3C with a Myc tag at the C terminus (N3C-Myc) was purified using an anti-Myc tag antibody from transfected cells, and the sequence of purified N3C-Myc was then analyzed by mass spectrometry (Fig. 2F). Peptides corresponding to amino acids (aa) 89 to 97 of N (AFVHGGVPR) were detected as the majority of the product, while no N-terminal peptides (aa 1 to 81) were observed, indicating that N3C lacks the N-terminal sequence and is started from aa 89 of N. Because the N terminus of N3C is not a methionine, it is considered that N3 was cleaved into N3C by a host protease or autoproteolysis.
N isoforms localize to distinct subcellular components.
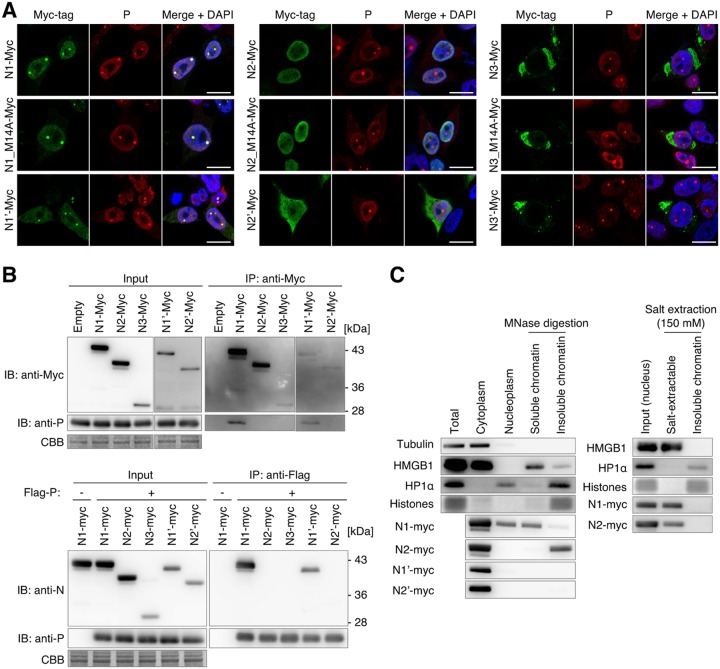
To understand the subcellular localization of N isoforms, we transfected N isoform expression plasmids in persistently BoDV-infected HEK293T (293T) cells and visualized each isoform by immunofluorescence analysis. We also transfected single-amino-acid substitution mutants of N1, N2, and N3, named N1_M14A, N2_M14A, and N3_M14A, which do not express N1′, N2′, and N3′, respectively, to exclude the possibility that the expression of N1′, N2′, and N3′ affects the localization of the full-length isoforms. As described above, N1 and N1′ mainly localize to the nucleus and accumulate at vSPOTs, the intranuclear replication sites of BoDV. As shown in Fig. 3A, N2 was not incorporated into vSPOTs despite its nuclear distribution. In contrast, N2′ was localized in the cytoplasm. Furthermore, transfection of N3 and N3′ expression plasmids, both of which induce the N3C isoform in cells (Fig. 2E), resulted in the distribution of the Myc tag signal only in the cytoplasmic component. Interestingly, an immunofluorescence assay using an ER marker revealed that the N3 isoform-accumulated component in the cytoplasm corresponded to the ER. These observations revealed that N isoforms show different subcellular localizations, indicating that N splicing may contribute to the distribution of protein isoforms in BoDV-infected cells. We confirmed that the presence of the isoforms initiated from the second AUG codon does not influence to the subcellular distribution of their full-length isoforms.
FIG 3.
Subcellular localization and P- and chromatin-binding abilities of N isoforms. (A) Localization of splicing isoforms with a C-terminal Myc tag. Persistently BoDV-infected 293T cells were transfected with plasmids encoding the indicated constructs, and each N protein was detected using an anti-Myc tag antibody. vSPOTs were stained by an anti-P antibody, and the nucleus was counterstained with DAPI. Bars, 10 μm. (B) Immunoprecipitation analysis of N isoforms. (Top) Immunoprecipitation (IP) of N isoforms and coimmunoprecipitation (co-IP) of P. (Bottom) IP of P and co-IP of N isoforms. Uninfected 293T cells were transfected with plasmids encoding the indicated N and P constructs. IP and co-IP of N isoforms and P were detected by Western blotting. CBB staining was used as a loading control. (C) Chromatin-binding assay of N isoforms. Uninfected 293T cells were transfected with plasmids encoding the indicated N isoforms. (Left) Transfected cells were fractionated into cytoplasm, nucleoplasm, and soluble and insoluble chromatin fractions using micrococcal nuclease (MNase) digestion. (Right) Nuclei of transfected cells were fractionated into salt-extractable and insoluble fractions with 150 mM NaCl. Tubulin, HMGB1, HP1α, and N isoforms were detected by Western blotting. CBB staining was used for detection of histones. For detection of tubulin, HMGB1, HP1α, and histones, lysates of N1-Myc-transfected cells were used.
We found that N2- and N3-derived isoforms did not localize to vSPOTs, probably due to a lack of interaction with viral P protein (12). Thus, the interaction between the N isoforms and P was investigated by immunoprecipitation (IP) analysis. As shown in Fig. 3B, P coimmunoprecipitated with N1 and N1′ but not with N2, N2′, and N3C. Consistently, N1 and N1′ coimmunoprecipitated with P, but N2, N2′, and N3C did not. A previous study demonstrated that aa 51 to 100 of N1 are important as a P-binding site (20). Because N2, N2′, and N3C lack a part of the P-binding sequences (Fig. 2A), the loss of P-binding ability may be critical for the vSPOT localization of these N isoforms.
We previously demonstrated that N1 binds to chromatin, allowing viral RNPs to segregate into daughter cell nuclei with host chromosomes (28). Therefore, we evaluated whether the nuclear isoforms of N conserve the chromatin-binding capacity. The chromatin fraction was prepared by subcellular fractionation, and the chromatin was digested with micrococcal nuclease (MNase), which yields soluble and insoluble chromatin. As described above, N1-Myc was detected in the soluble chromatin fraction where HMGB1 was observed, while N1′-Myc was not. Interestingly, N2-Myc was not observed in the soluble chromatin fraction but was detected with insoluble chromatin (Fig. 3C). This suggested that the preferences for the binding region on chromatin may be different between N1 and N2. To test the binding affinity for chromatin, we extracted chromatin-binding proteins using salt extraction. As shown in Fig. 3C, both N1 and N2 were extracted at a salt concentration of 150 mM, which is a relatively low salt concentration, suggesting that the binding affinities of N1 and N2 are relatively weak.
The N3 isoform translocates to the ER and is cleaved by host signal peptidase.
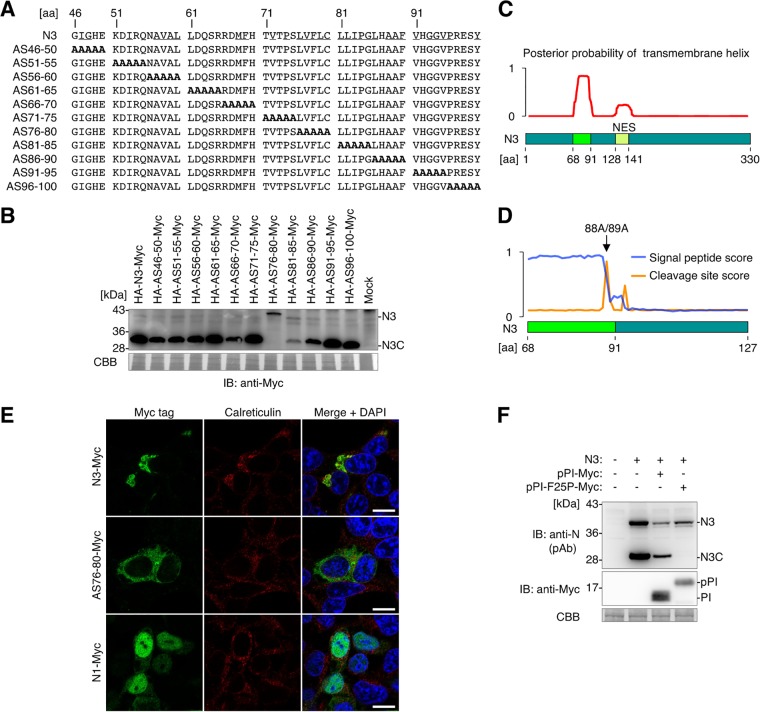
To identify the mechanism of N3 posttranslational modification that produces N3C, which accumulates in the ER, alanine-scanning mutational analysis of N3 was performed (Fig. 4A). Alanine replacement of aa 76 to 80 (AS76-80 mutant) completely abolished the expression of N3C, indicating that the 76LVFLC80 region is essential for N3C expression (Fig. 4B).
FIG 4.
N3 translocates to the ER and is cleaved into a C-terminal fragment. (A) Protein sequences of N3 mutants used for alanine-scanning mutagenesis analysis. Underlined characters in the N3 sequence represent amino acids with hydrophobic residues. (B) Detection of alanine-scanning N3 mutants by Western blotting. Whole-cell lysates of uninfected 293T cells expressing each mutant were used. (C) Detection of transmembrane helix potential by TMHMM software, which predicts transmembrane helices in proteins. Full-length N3 (aa 1 to 330) was used for analysis. (D) Detection of signal peptide potential by SignalP, which predicts the presence and location of the signal peptide cleavage site in proteins. A partial N3 sequence (aa 68 to 127) was used for analysis. (E) Cellular localization of N3-Myc, AS76-80–Myc, and N1-Myc in uninfected 293T cells. Each N protein was detected by an anti-Myc tag antibody. The ER was stained by an anticalreticulin antibody, and the nucleus was counterstained with DAPI. Bars, 10 μm. (F) N3 is cleaved into N3C by the host SPase. N3C expression was detected by Western blotting. Whole-cell lysates of uninfected 293T cells expressing the indicated constructs were used. pPI-Myc, preproinsulin with a C-terminal Myc tag; pPI-F25P-Myc, pPI-Myc with a single-amino-acid substitution, which functions as an SPase inhibitor. In panels B and F, CBB staining was used as a loading control.
The region around aa 76 to 80 is highly hydrophobic, raising the possibility that the sequence around 76LVFLC80 may contain the transmembrane domain (Fig. 4A). TMHMM software (29), which predicts transmembrane helices in proteins, demonstrated that the hydrophobic sequence from aa 68 to 91 exhibited a high probability of being a transmembrane domain (Fig. 4C). Furthermore, SignalP (30), which predicts the presence and location of signal peptide cleavage sites in proteins, revealed that the sequence from aa 68 to 88 was a high-scoring signal peptide and that N3 is predicted to be cleaved between A88 and A89 by host signal peptidase (SPase) (Fig. 4D). The predicted cleavage site was at the same position as that of the N terminus of N3C (Fig. 2F), suggesting that N harbors the intrinsic ER-targeting sequence and translocates to the ER, followed by the cleavage of the signal peptide by host SPase.
To test this hypothesis, the localization of N3 was investigated by immunofluorescence analysis. N3-Myc colocalized with calreticulin, an ER protein (Fig. 4E), in uninfected 293T cells. In contrast, the AS76-80 mutant did not localize to the ER, but it was diffusely detected in the cytoplasm. These observations demonstrated that N3 translocates to the ER and that the sequence around 76LVFLC80 acts as the ER-targeting signal peptide. To determine if N3 is cleaved into N3C by the host SPase, a competitive SPase inhibition assay was performed. Preproinsulin (pPI) is a secretory preprotein that translocates to the ER, where it is cleaved into proinsulin (PI). A mutant of pPI (pPI-F25P), which harbors a single-amino-acid substitution following the signal peptide cleavage site, specifically binds to SPase catalytic subunits and acts as a competitive inhibitor of cellular SPase (31). As shown in Fig. 4F, N3C was expressed when N3 was coexpressed with wild-type pPI, but the expression of N3C was completely abolished when the host SPase was inhibited by pPI-F25P, demonstrating that N3C is cleaved by host SPase. Taken together, these findings demonstrated that the intrinsic ER-targeting signal peptide around aa 76 to 80 is the molecular determinant for N3 to translocate to the ER, where it is cleaved by the host SPase.
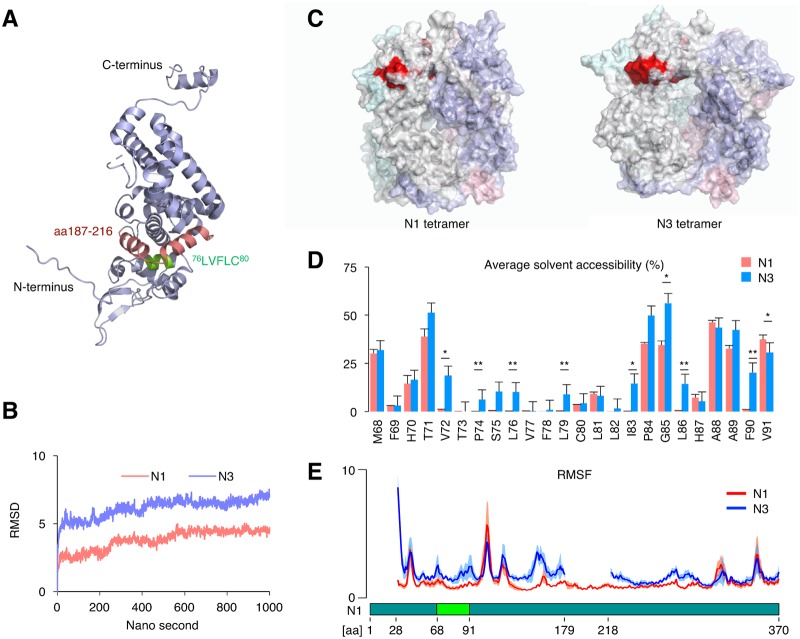
The N3 isoform signal peptide is predicted to be exposed to solvent.
Although both N1 and N3 harbor signal peptides, only N3 translocates to the ER, suggesting that NI-II splicing is a molecular switch for ER targeting. However, it is unclear how the removal of the NI-II intron is the ER-targeting signal on N3. The crystal structure of N1 showed that the region between aa 76 and 80 is embedded inside the molecule and that the region between aa 187 and 216 structurally covers 76LVFLC80 (Fig. 5A). Given that the cover sequence within aa 187 to 216 is removed by NI-II splicing, it may unmask the intrinsic ER-targeting signal peptide and make it available for interaction with the ER translocation machinery. To test this hypothesis, structural simulation of the N1 tetramer and N3 tetramer was performed by molecular dynamics (MD) analysis using the previously reported crystal structure of the N1 tetramer (32) to observe if the ER-targeting signal peptide is exposed to the protein surface by NI-II splicing (Fig. 5B). The predicted structure of the N3 tetramer was different from that of the N1 tetramer (Fig. 5C). Although the solvent accessibility of atoms around 76LVFLC80 was quite low in N1, it markedly increased in N3 (Fig. 5D). Furthermore, atomic fluctuation around 76LVFLC80 and the N terminus was also increased in N3 (Fig. 5E). The crystal structure of the N1 tetramer suggested that the region from aa 179 to 218 plays an important role in the interaction with the N terminus of the neighboring monomer. The increased atomic fluctuation of the N3 tetramer supports that the region from aa 179 to 218 is crucial for forming the stable tetramer. These results suggested that NI-II splicing makes the targeting signal accessible to the ER translocation machinery via a conformational change and partial exposure to the protein surface.
FIG 5.
The ER-targeting signal peptide is partially exposed to the protein surface in the predicted N3 structure. (A) Crystal structure of N1 (PDB accession number 1N93). Green and red regions represent 76LVFVC80 and aa 187 to 216, respectively. (B) Root mean square deviation (RMSD) of the N1 tetramer and N3 tetramer. (C) Predicted structures of the N1 tetramer and N3 tetramer. The red region represents 76LVFVC80. (D) Average solvent accessibility of each residue from aa 68 to 91 in N1 and N3. The data are presented as the means and standard errors of the means (SEM) of data from four independent simulations. Student’s t test was used for statistical analysis. *, P < 0.05; **, P < 0.01. (E) Root mean square fluctuation (RMSF) of each residue in N1 and N3. The data are presented as the means ± 95% confidential intervals (CIs) of data from four independent simulations. Solid lines show means, and shaded regions represent CIs.
Expression of N3C in the ER fraction of BoDV-infected cells.
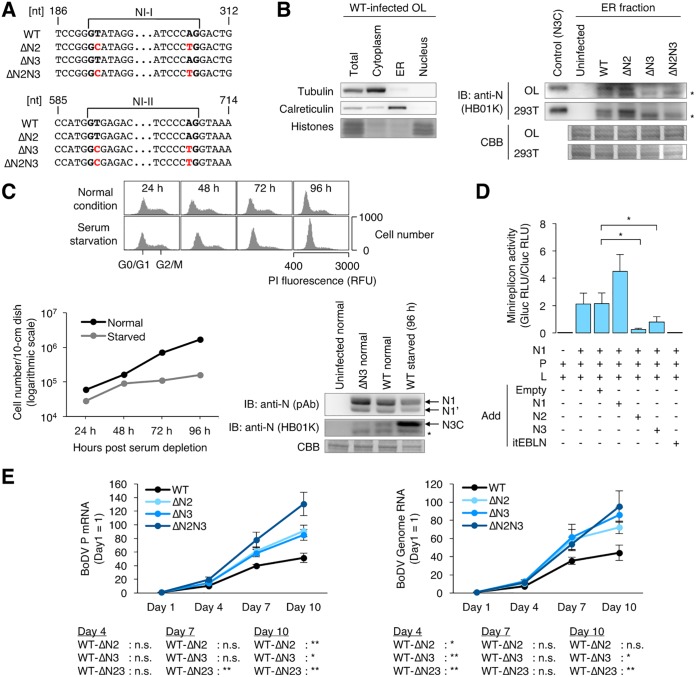
To elucidate if N2 and N3C are expressed in BoDV-infected cells, rBoDVs, which harbor silent mutations at splicing donor and acceptor sites within N, were generated (Fig. 6A). The successful introduction of silent mutations was verified by RT-PCR and sequencing of the rBoDV genome (data not shown). To detect the ER-specific expression of N3C, rBoDV-infected OL, 293T, and Vero cells were fractionated into the cytoplasm, ER, and nucleus. As shown in Fig. 6B, N3C was detected in the ER fraction of OL and 293T cells by Western blotting, although the expression of N3C was not detected in Vero cells (data not shown). These results confirmed that N3C is expressed in the ER during persistent infection by BoDV.
FIG 6.
Splicing of N isoforms negatively regulates BoDV infection. (A) Partial sequences of rBoDVs. Silent mutations that disrupt splicing signals in the N gene are shown in red. (B) Expression of N3C in BoDV-infected cells. Uninfected or persistently rBoDV-infected OL and 293T cells were fractionated into cytoplasm, ER, and nucleus fractions. The ER fraction was used for detection of N3C. The whole-cell lysate of uninfected 293T cells transfected with an N3-expressing plasmid was used for the positive control. N3C, tubulin, and calreticulin were detected by Western blotting. CBB staining was used as a loading control and for detection of histones. Asterisks represent an unidentified N product. (C) Expression levels of N isoforms during serum starvation. Persistently BoDV-infected OL cells were cultured in serum-free medium. (Top and bottom left) Distribution of cell cycles and cell numbers after serum starvation, respectively. For cell cycle detection, the cells were stained by propidium iodide (PI). (Bottom right) Expression levels of the N isoforms. N1, N1′, and N3C were detected by Western blotting. The whole-cell lysate was used for N1 and N1′, and the ER fraction was used for N3C detection. CBB staining was used as a loading control. Normal and starved represent cells cultured in serum-containing and serum-free media, respectively. The asterisk represents an unidentified N product. RFU, relative fluorescence units. (D) Expression of N2 and N3 inhibits BoDV polymerase activity in the minireplicon assay. Uninfected 293T cells were transfected with plasmids encoding the indicated proteins, and luciferase activity was measured at 48 h posttransfection. Gluc (Gaussia luciferase) activity, which was derived from the BoDV minireplicon, was normalized to Cluc (Cypridina luciferase) activity, which was derived from a transfection control plasmid. The data are presented as the means and SEM of results from three independent experiments. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were used for statistical analysis. *, P < 0.05. RLU, relative light units. (E) Propagation of rBoDVs. OL cells were infected with rBoDVs at an MOI of 0.1. The levels of BoDV P mRNA and genome RNA at the indicated time points were measured by qRT-PCR. The data are presented as the means ± SEM of results from three independent experiments. One-way ANOVA and Tukey’s post hoc test were used for statistical analysis. *, P < 0.05; **, P < 0.01; n.s., not significant.
On the other hand, the expression of N2 and N2′ could not be evaluated in BoDV-infected cells, suggesting that the expression of N2 and N2′ is masked by the signal of N1′. The expected molecular weights of N2 and N2′ (approximately 34 to 36 kDa) were close to that of N1′ (38 kDa), and the splicing efficiency of NI-I was quite low. Further optimization of Western blotting is required to evaluate the expression of NI-I splicing products.
The primary target of BoDV in natural infection is neurons. Neurons are nonproliferating cells; therefore, we evaluated whether the expression levels of N isoforms depend on cell proliferation. BoDV-infected OL cells cultured in serum-free medium showed reduced proliferation and cell cycle arrest 72 and 96 h after serum depletion (Fig. 6C), suggesting that quiescence was induced. While the expression of N1 and N1′ did not show obvious changes by serum starvation, the expression of N3C appeared to increase (Fig. 6C). This suggested that the expression of N3C may be regulated by cell proliferation.
N2 and N3 negatively regulate BoDV infection.
The roles of N isoforms in BoDV replication were investigated. A minireplicon system of BoDV, which synthesizes recombinant BoDV nucleocapsids containing a minigenome reporter RNA, was used following transfection of expression plasmids encoding N, P, L, and the minigenome. The minireplicon assay was performed in the presence or absence of plasmids expressing N1, N2, and N3. Consistent with previous results, viral polymerase activity was strongly inhibited when itEBLN (an endogenous bornavirus-like nucleoprotein [EBLN] in the thirteen-lined ground squirrel genome) (33) was cotransfected with the minireplicon constructs. Although N1 did not inhibit the polymerase activity of the minireplicon, N2 and N3 significantly decreased the polymerase activity in the system (Fig. 6D). These results suggested that N2 and N3 act as a negative inhibitor of viral transcription and replication.
To understand the role of NI-I and NI-II splicing in viral replication, the propagation of rBoDV lacking NI-I splicing, NI-II splicing, or both was investigated. OL cells were infected with rBoDVs, and the levels of viral mRNA and genome RNA in cells were monitored. As shown in Fig. 6E, levels of both viral mRNA and genome RNA were significantly increased in cells infected with splicing-deficient rBoDVs compared to wild-type rBoDV. These results suggested that the expression of N2 and N3 negatively regulates viral polymerase activity during BoDV infection.
Conservation of the splicing signals and hydrophobic region of N across orthobornaviruses and endogenous bornavirus-like nucleoproteins.
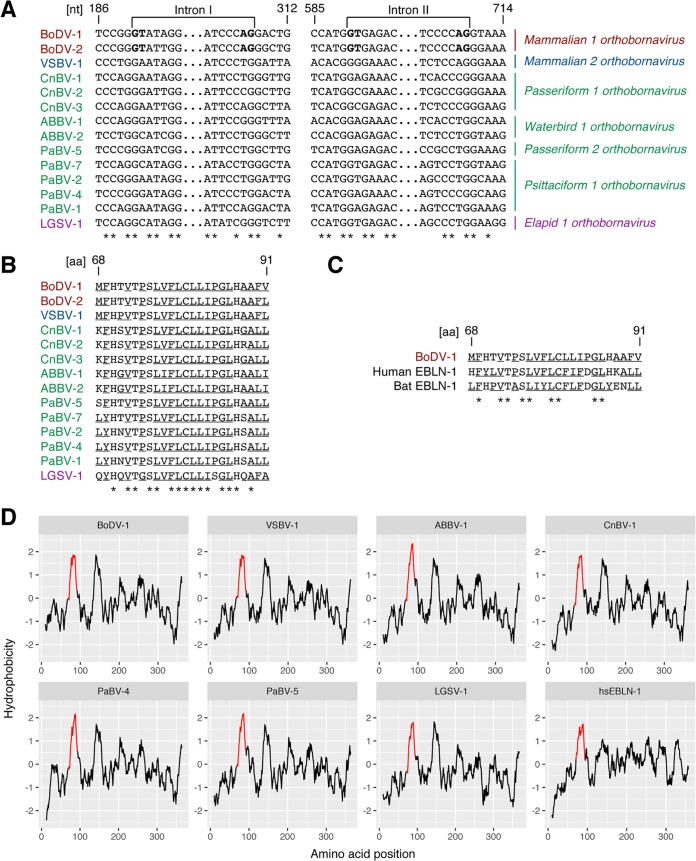
To elucidate if the splicing acceptor/donor sites are conserved in orthobornaviruses, the N genes of orthobornaviruses were aligned. While the splicing signals were conserved across mammalian 1 orthobornaviruses, other orthobornaviruses did not have the splicing signals in the corresponding regions (Fig. 7A). The hydrophobic transmembrane region was aligned in orthobornaviruses. All orthobornavirus N proteins contained many hydrophobic residues in the regions corresponding to the predicted transmembrane domain of N3 (Fig. 7B and D). These results suggested that the hydrophobic transmembrane potential is a conserved feature of orthobornaviruses.
FIG 7.
Comparison of splicing signals and hydrophobic regions across orthobornaviruses and endogenous bornavirus-like nucleoproteins. (A) Comparison of the splicing signals across orthobornaviruses. Nucleotide positions corresponding to the BoDV-1 genome are shown. Splicing signals are shown in boldface type. Asterisks represent conserved nucleotides. (B) Conservation of the signal peptide across orthobornaviruses. Amino acid positions corresponding to BoDV-1 N1 are shown. Underlined characters represent amino acids with hydrophobic residues. Asterisks represent conserved amino acids. (C) Conservation of the hydrophobic region in human and bat EBLN-1. Amino acid positions corresponding to BoDV-1 N1 are shown. Underlined characters represent amino acids with hydrophobic residues. Asterisks represent conserved amino acids. (D) Hydrophobicity of nucleoproteins across orthobornaviruses and an endogenous bornavirus-like element. Hydrophobicity was calculated by ProtScale. Full-length nucleoproteins of orthobornaviruses and endogenous bornavirus-like nucleoproteins were used for analysis. The regions corresponding to the hydrophobic transmembrane domain of N3 are shown in red. Accession numbers of protein sequences used for analysis are as follows: P0C796 for BoDV-1, YP_009269413 for variegated squirrel bornavirus 1 (VSBV-1), YP_009237642 for aquatic bird bornavirus 1 (ABBV-1), YP_009268905 for canary bornavirus 1 (CnBV-1), YP_009268893 for parrot bornavirus 4 (PaBV-4), YP_009268899 for PaBV-5, YP_009055058 for Loveridge’s garter snake virus 1 (LGSV-1), and NP_001186867 for Homo sapiens EBLN-1 (hsEBLN-1).
To elucidate if such sequence features of orthobornavirus N are evolutionarily conserved, the sequences of EBLNs, which are the ancient bornaviral sequences in host genomes, were investigated. EBLNs are remnants of ancient viruses, and they provide useful information for understanding the characteristics of ancient viruses. Although most of the ORFs of integrated N sequences were disrupted by mutations, two EBLNs in the human genome and bat genome retained almost intact ORFs (34, 35). Interestingly, both EBLNs contained hydrophobic regions, suggesting that some antient bornaviruses might have already acquired the transmembrane potential of N (Fig. 7C).
DISCUSSION
The present study demonstrated that BoDV generates multiple N isoforms by mechanisms such as downstream translation initiation and mRNA splicing (Fig. 1 and 2). The newly identified splicing in N had canonical eukaryotic splicing site sequences, suggesting that BoDV uses host splicing machinery, as previously reported (23, 24). The expression levels of the N spliced transcripts were relatively low at less than 7% of the N transcripts in BoDV-infected cell lines by the estimation of NGS data. Previous data suggested that BoDV harbors an exon splicing suppressor sequence and controls alternative splicing of L transcripts (25, 36). Thus, it may be possible that BoDV also controls the efficiency of N splicing to maintain the expression of N isoforms at low levels. In this study, we could not detect N3C in Vero cells, while it was detected in OL and 293T cells. Furthermore, the expression of N3C increased in nonproliferating cells (Fig. 6). It would be of interest to determine how the expression level of N3C is controlled in cells. It may also be necessary to estimate the expression levels of N isoforms in BoDV-infected animal brains to understand the role of N splicing in vivo.
Many viruses replicating in the nucleus employ RNA splicing machinery to create variant proteins from a single transcript, which generally play different roles in viral infection. Because genome sizes and coding capacities are relatively limited in nuclear-replicating RNA viruses, RNA splicing may be a conducive strategy to produce viral protein diversity. For instance, influenza A virus expresses isoforms of NS and M proteins using the host splicing machinery (37). It has been reported that the splicing efficiency of the NS transcript coordinates the cellular antiviral response and the nuclear export of viral RNPs (38). In addition, the splicing of the M transcript generates an ion channel protein required for virus particle assembly, egress, and ingress, in addition to the structural matrix protein (39). Furthermore, it has been reported that the mosquito Culex tritaeniorhynchus rhabdovirus requires mRNA splicing to express mature L protein, similarly to BoDV (40).
The utilization of alternative AUG start codons in the same transcript is another mechanism to express multiple protein isoforms of different functions in many RNA viruses. Ribosomal leaky scanning and ribosomal shunts are common mechanisms for the P transcript of Mononegavirales. For example, the Sendai virus P transcript expresses as many as five different isoforms using alternative AUG start codons, namely, P, C, C′, Y1, and Y2 (41), suggesting that alternative usage of start codons may be a useful mechanism for RNA viruses replicating in the cytoplasm. The present study showed that mRNA splicing and alternative start codon usage cooperate for the expression of N isoforms showing diverse subcellular localizations in BoDV-infected cells. This may be the first report of the coordination between posttranscriptional and translational regulations to create protein diversity in RNA virus infection.
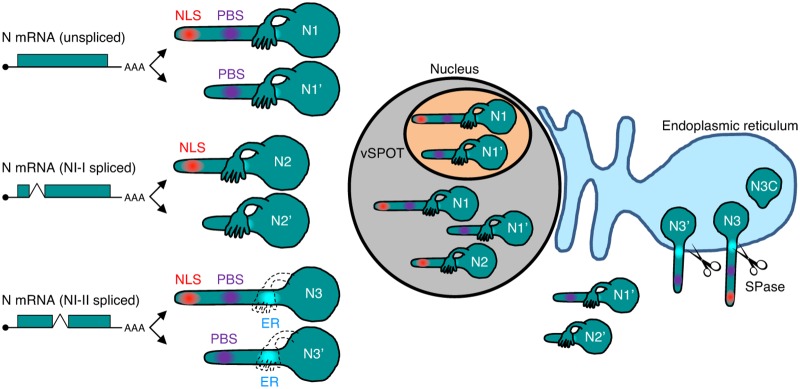
The present study showed that the subcellular localizations of BoDV N isoforms are elaborately controlled by their signal motifs within the sequence, such as the P-binding site, NLS, NES, and ER-targeting signal peptides (Fig. 3 and 8). The full-length N1 isoform mainly localizes to the nucleus and accumulates in vSPOTs. N1′, which lacks an NLS, localizes in the cytoplasm but translocates to the nucleus and accumulates in vSPOTs via interaction with P. Although the spliced N2 isoform contains an NLS and localizes to the nucleus, it does not accumulate at vSPOTs due to the lack of the P-binding site (Fig. 8). N2′ lacks both the NLS and P-binding site and, therefore, cannot enter the nucleus. NI-II splicing generates N3 and N3′, which translocate to the ER, resulting in the development of a truncated form of N3C (Fig. 8). The isoforms produced from a transcript showing different intracellular localizations comprise a unique mechanism for BoDV.
FIG 8.
Schematic representation of N isoforms and their subcellular localization during BoDV infection. N1, N2, and N3 mRNAs are transcribed from the N gene. Each mRNA generates two isoforms by translation initiation from the first and second AUG codons. N1 translocates to the nucleus and accumulates in vSPOTs. The sole expression of N1′ localizes to the cytoplasm, while N1′ can enter the nucleus and vSPOTs via interaction with N1 and P in infected cells (Fig. 3A). N2 is transported into the nucleus, but N2 does not localize in vSPOTs. N2′ localizes only in the cytoplasm. N3 and N3′ translocate to the ER and are cleaved into the N3C C-terminal fragment by the host SPase.
Among the N isoforms, N3 and N3′ may regulate processing and translocation in infected cells. N3 and N3′ translocate to the ER and are cleaved in the C-terminal domain to generate N3C. The expression of N3 isoforms was detected in the ER fraction of rBoDV-infected cells by Western blotting (Fig. 6). Transfection analysis revealed that N3C was more abundant than N3, suggesting that a large portion of N3 in the ER is cleaved by the host SPase. Although N3 and N3C contain an NLS at the N terminus, most of the signals of these isoforms were detected at the ER and not in the nucleus. Structural prediction of N3 revealed that the N terminus of N3 is more flexible than that of N1, suggesting that the flexible N terminus of N3 isoforms contributes to escape from the recognition of importin. Alternatively, the flexibility of the N terminus might affect the oligomerization of N3, thereby preventing the nuclear localization of N3 isoforms.
Another interesting mechanism is the activation of the ER-targeting signal on N3 isoforms. ER targeting of N3 is accomplished by canonical ER translocation machinery. The sequence from aa 68 to 91 contains several hydrophobic residues and is predicted to form a helical structure (Fig. 4), suggesting that this region is a transmembrane domain. In silico prediction and alanine substitution analyses demonstrated that this region functions as a signal peptide that binds to the translocon in the ER membrane. Although N1 also has this signal peptide, only N3 translocates in the ER. It is unknown why N1 is not recognized by the ER translocation machinery. The prediction assay of the N3 structure revealed that the signal peptides in N3 exhibit higher solvent accessibility than N1 (Fig. 5), suggesting that the region from aa 179 to 218 (region deleted by N-II splicing) masks the signal peptides in N1 (Fig. 8). Furthermore, N3 has a more flexible structure than N1. This finding demonstrated that N3 may be highly susceptible to conformational change following interaction with the translocation machinery in cells.
The minireplicon assay and infection experiment using rBoDVs lacking N splicing showed that N2 and N3 isoforms negatively regulate viral transcription and replication (Fig. 6). N2 strongly inhibited the polymerase activity of the minireplicon, whereas N3 moderately inhibited the polymerase activity of the minireplicon. Because N2 cannot interact with P and does not accumulate in vSPOTs, this isoform may act as a decoy for host factors that are important for BoDV replication. Although the inhibition of minireplicon activity by N3 was not as strong as that by N2, rBoDV-ΔN3 propagated as efficiently as rBoDV-ΔN2. Considering that N3C localized to the ER, it is likely that N3 plays a negative role in BoDV replication within the intracellular organelle. The finding that the expression level of N3 increased during serum starvation suggested that N3C may downregulate BoDV replication depending on the extent of cell proliferation. BoDV N is a multifunctional protein that plays a central role in viral infection, such as in viral RNP formation and nuclear transport of viral RNPs, as well as in inhibition of host immune responses (13, 42–45). Although the present study could not demonstrate the precise mechanisms of spliced N isoforms regulating BoDV replication, the distinct cellular distributions of N may control and maintain a unique persistent infection by BoDV in the nucleus. Further experiments, such as gene expression profiling and identification of protein interactions, are required to understand the impact of N isoforms on both viral and cellular functions.
The present data showed that the N proteins of other orthobornaviruses also have many hydrophobic residues in the region corresponding to the transmembrane domain of N3 (Fig. 7), suggesting that these proteins also exhibit transmembrane potential in infected cells. Intriguingly, human EBLN-1, which originated from an ancestor of orthobornavirus approximately 45 million years ago, also contains hydrophobic residues in the corresponding region, suggesting that some antient bornaviruses might have already acquired transmembrane potential. According to analysis by TMHMM (data not shown), the fact that nucleoproteins of other negative-strand RNA viruses show little or no transmembrane potential suggested that the ER targeting of N might be a strategy of orthobornaviruses crucial for their unique characteristics, such as intranuclear replication and persistent infection.
In conclusion, the present study demonstrated that functions of BoDV N isoforms are controlled by their subcellular localizations determined by splicing and downstream AUG initiation. Such unique features of N, in addition to intranuclear persistent infection and noncytolytic replication, may distinguish BoDV from other negative-strand RNA viruses. Further experiments on the mechanisms of N2 and N3 inhibition of BoDV replication as well as the control of diverse subcellular localizations will aid in the understanding of the unique characteristics of BoDV.
MATERIALS AND METHODS
Cell lines and viruses.
OL (human oligodendroglioma) cells were cultured in high-glucose (4.5%) Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS). HEK293T (293T) (human embryonic kidney) and Vero (green monkey kidney) cells were cultured in low-glucose (1.0%) DMEM supplemented with 10% and 2% FBS, respectively. BoDV-infected OL cells, a cell line persistently infected with strain huP2br (46), was cultured under the same conditions as the parental cell line. BoDV-infected OL, 293T, and Vero cells, cell lines persistently infected with strain He/80/Fct (He/80/FR harboring a single-nucleotide substitution from C to T at genome position 4673) or rBoDV, were cultured under the same conditions as the parental cell lines.
Cell-free virus preparation.
Cell-free BoDV was prepared as previously described (47). Briefly, BoDV-infected cells were treated with 0.05% trypsin and 0.48 mM EDTA and centrifuged. After centrifugation, the cells were washed once with phosphate-buffered saline (PBS) and suspended in Opti-MEM (ThermoFisher) or DMEM containing 2% FBS. The suspended cells were sonicated and centrifuged at 1,200 × g for 25 min at 4°C. The supernatant containing viral materials was stored at −80°C until use.
Virus infection.
For amplicon sequencing, OL cells were infected with He/80 at a multiplicity of infection (MOI) of 1. After absorption for 1 h, the cells were washed with PBS. For propagation assays, OL cells were infected with either rBoDV-WT, rBoDV-ΔN2, rBoDV-ΔN3, or rBoDV-ΔN2N3 at an MOI of 0.1. After absorption for 1 h, the cells were washed with PBS and passaged at 1, 4, 7, and 10 days postinfection. After 10 days postinfection, the cells were passaged within the appropriate interval of a few days. Virus propagation was detected by immunofluorescence analysis of P.
Plasmid construction.
To generate the eukaryotic expression plasmid of N1 (pcDNA3-N1), the PCR-amplified BoDV N1 coding DNA sequence (CDS) fragment was cloned into the plasmid pcDNA3 (Invitrogen). The N1 gene was amplified from cDNA from the cells infected with BoDV strain He/80/Fct. The expression plasmids of N isoforms were constructed from pcDNA3-N1 by PCR mutagenesis to delete the intron in focus and introduce the synonymous point mutations in another splicing signal. A Myc tag, a Flag tag, and a hemagglutinin (HA) tag were fused to the 5′ end or the 3′ end of the CDS by PCR mutagenesis. To generate the expression plasmid of P with an N-terminal Flag tag (pcDNA3-Flag-P), the PCR-amplified BoDV P CDS fragment from pCXN2-P (48) was cloned into the plasmid pcDNA3. A Flag tag was fused to the 5′ end of the CDS by PCR mutagenesis. The RNA polymerase II (Pol II)-driven BoDV minigenome plasmid pFct-BDV was described previously (49). pFct-BDV_ΔN2, pFct-BDV_ΔN3, and pFct-BDV_ΔN2N3 were constructed from pFct-BDV by PCR mutagenesis to introduce the point mutations in the splicing signals in the N gene. To obtain the eukaryotic expression plasmids of pPI (pCAG-pPI-Myc) and pPI-F25P (pCAG-pPI-F25P-Myc), the PCR-amplified pPI gene was cloned into the plasmid pCAGGS.MCS. A Myc tag was fused to the 3′ end of the pPI CDS by PCR mutagenesis. pCAG-pPI-F25P-Myc was constructed from pCAG-pPI-Myc by PCR mutagenesis to introduce the point mutation.
BoDV reverse genetics.
Reverse-genetics assays were carried out based on data from a previous study (49). Briefly, 293T cells were transfected with either the pFct-BDV, pFct-BDV_ΔN2, pFct-BDV_ΔN3, or pFct-BDV_ΔN2N3 plasmid and pCA-N, pCXN2-P, and pCAGGS-L using polyethylenimine max (linear; MW, 25,000) (Polysciences, Inc.). Three days after transfection, the cells were passaged and cocultivated with Vero cells. The Vero cells were passaged every 3 days, and the rescue efficacy of recombinant BoDV was evaluated by immunofluorescence analysis of P.
Minigenome assay.
A minigenome assay was performed according to a protocol described previously (50). Briefly, 293T cells were transfected with a Pol II-driven minigenome plasmid carrying the Gaussia luciferase gene; helper plasmids expressing the BoDV-1 N1, P, and L genes; and a control plasmid expressing the Cypridina luciferase gene using polyethylenimine max. Forty-eight hours after transfection, Gaussia luciferase activity was measured with a BioLux luciferase assay kit (NEB) and normalized to the corresponding Cypridina luciferase activity.
Subcellular fractionation.
293T cells (7.5 × 106) were resuspended in 120 μl cold HMKE buffer (20 mM HEPES [pH 7.2], 5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 250 mM sucrose, 400 μg/ml digitonin, protease inhibitor), followed by pulsed vortexing and incubation on ice for 5 min. The lysates were centrifuged in Microfuge tubes at 1,000 × g for 3 min at 4°C. The supernatant from this process represents the cytoplasmic fraction. The pellets were gently resuspended in NP-40 buffer (0.5% NP-40, 1× PBS, protease inhibitor), followed by pulsed vortexing and incubation on ice for 1 min. The lysates were centrifuged for 3 min at 1,000 × g at 4°C. The supernatant and pellet from this spin represent the ER and nuclear fractions, respectively. For the fractionation of OL cells, the same protocol for 293T cells with one modification (HMKE buffer containing 800 μg/ml digitonin) was used.
Serum starvation.
OL cells (1 × 104 cells) were seeded into 10-cm dishes and incubated for 24 h in medium containing 5% FBS. The medium was removed, and the cells were washed four times with fresh serum-free medium. The cells were then cultured in serum-free medium for 24 to 96 h. Ninety-six hours after serum starvation, cells were collected, and the ER fraction was prepared using the same protocol as the one described above. For propidium iodide (PI) staining, cells were collected 24, 48, 72, and 96 h after serum depletion and stained with a Tali cell cycle kit. The fluorescence of PI and the cell number were measured using a Tali image cytometer.
Chromatin-binding assay (MNase digestion).
Cells (2.5 × 106 cells) were resuspended in 100 μl buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM dithiothreitol [DTT], protease inhibitor) containing 0.1% Triton X-100. After incubation for 10 min on ice, nuclei were collected in a pellet by centrifugation (5 min at 1,200 × g). Nuclei were washed three times with buffer A and lysed in 100 μl buffer B (3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease inhibitor) for 30 min. The chromatin-enriched pellet was collected by centrifugation (5 min at 1,700 × g), and the supernatant was removed to yield a nucleoplasmic fraction. The pellet was washed twice with buffer B and once with MNase buffer (20 mM Tris-HCl [pH 8.0], 5 mM NaCl, 2.5 mM CaCl2) and resuspended in 100 μl MNase buffer containing 20 U MNase. After incubation for 5 min at 37°C, the MNase reaction was stopped by the addition of an EDTA-EGTA solution to a final concentration of 5 mM, and the supernatant was then collected by centrifugation (5 min at 2,000 × g) as a chromatin-binding fraction. The pellet was washed three times with MNase buffer and collected as an insoluble fraction. All steps were carried out at 4°C if the condition was not specified.
Chromatin-binding assay (salt extraction).
Nuclei were prepared using the same protocol as the one for the chromatin-binding assay with MNase. Nuclei were washed three times with buffer A and lysed in buffer A containing 150 mM NaCl. Nuclei were incubated for 30 min and centrifuged for 1 h at 21,500 × g. The supernatant and pellet from this process represent salt-extractable and insoluble fractions, respectively. All steps were carried out at 4°C.
Western blotting.
The cell lysates were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with Tris-buffered saline (TBS)–0.1% Tween 20 with 5% (wt/vol) low-fat milk powder (TBSTM) and then reacted with anti-N (HB01 or HB01K), anti-P (HB03), anti-Myc (9E10 [Millipore] or 9B11 [Cell Signaling]), anti-Flag M2 (Sigma-Aldrich), antitubulin (B-5-1-2; Sigma-Aldrich), anticalreticulin (ab92516; Abcam), anti-HMGB1 (ab18256; Abcam), and HP1α (catalog number 2616; Cell Signaling) antibodies, which were diluted appropriately in TBSTM, followed by a reaction with horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch). The protein bands were visualized with the ECL Prime Western blot detection reagents (GE Healthcare).
Immunofluorescence analysis.
Persistently BoDV-infected 293T cells were transfected with the plasmids expressing the N isoforms and incubated for 24 h. The cells were fixed in 4% paraformaldehyde (PFA), permeabilized by incubation in PBS containing 0.25% Triton X-100, and then blocked with PBS containing 1% bovine serum albumin (BSA). Uninfected 293T cells were transfected with the plasmids expressing the N isoforms and the N3 mutant and incubated for 24 h. The cells were fixed in 100% methanol, because the anticalreticulin antibody was available only in the cells fixed in methanol. Methanol-fixed cells were blocked with PBS containing 1% BSA. Both PFA- and methanol-fixed cells were then incubated with the anti-BoDV P (HB03), anti-Myc (9E10), and anticalreticulin (ab92516) antibodies. This was followed by incubation with the appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen). The cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole). An Eclipse Ti confocal laser scanning microscope (Nikon) was used for data collection.
Immunoprecipitation.
293T cells were transfected with pCXN2-P (48) and pcDNA3 plasmids encoding Myc-tagged N isoforms for precipitation of N isoforms. 293T cells were transfected with pcDNA3-Flag-P and pcDNA3 plasmids encoding Myc-tagged N isoforms for precipitation of P. At 48 h posttransfection, the cells were collected and washed with PBS twice. The cells were lysed with lysis buffer (1× PBS, 0.5% NP-40, protease inhibitor). After centrifugation (3,000 × g for 3 min), the supernatant was collected. Protein G Dynabeads (Invitrogen) were incubated with anti-Myc tag antibody My3 (MBL) or anti-Flag tag antibody FLA-1 (MBL) and incubated for 10 min with rotation. The beads were washed with lysis buffer once, and the supernatant was added. After incubation with gentle rocking for 2 h, the beads were washed with lysis buffer three times, and the immunoprecipitated proteins were eluted with the Myc tag peptide or the 3×Flag tag peptide. All steps were carried out at 4°C.
Purification of Myc-tagged N3C.
293T cells were transfected with the pcDNA3-N3-Myc plasmid using polyethylenimine max. At 48 h posttransfection, the cells were collected and washed with PBS twice. The cells were lysed with lysis buffer (1× PBS, 0.5% NP-40, protease inhibitor). After centrifugation (3,000 × g for 3 min), the supernatant was collected. Protein G Dynabeads were incubated with anti-Myc tag antibody 9E10 and incubated with rotation for 10 min. The beads were washed with lysis buffer once, and the supernatant was added. After incubation with gentle rocking for 2 h, the beads were washed with lysis buffer three times, and the immunoprecipitated protein was eluted with the Myc tag peptide. All steps were carried out at 4°C.
Antigen-specific antibody purification.
The peptide corresponding to aa 331 to 370 of N1 conjugated with 5 cysteines at the N terminus was synthesized. The C-terminus-specific polyclonal anti-N antibody (HB01K) was purified from the polyclonal anti-N antibody (HB01) with the peptide using a high-affinity antibody purification kit (GenScript).
qRT-PCR analysis.
Total RNAs were extracted using the NucleoSpin RNA kit (Macherey-Nagel). Reverse transcription was performed using the Verso cDNA synthesis kit (ThermoFisher). RT-quantitative PCR (qRT-PCR) analyses were carried out using the Thunderbird SYBR qPCR mix and the Thunderbird probe qPCR mix (Toyobo) as previously described (50).
RNA-seq and data processing.
The sequences of mRNAs from uninfected and He/80/Fct-infected OL cells were analyzed by NGS. Total RNA was extracted using TRIzol reagent (Invitrogen) and the Direct-zol RNA MiniPrep kit (Zymo Research). The qualities of RNA samples were checked with a high-sensitivity RNA kit (Agilent). Next, poly(A)+ RNA was extracted using the Dynabeads mRNA purification kit (ThermoFisher). An NGS library was prepared using TruSeq stranded total RNA with the Ribo-Zero Gold LT sample prep kit (Illumina) according to the manufacturer’s instructions, except for the Ribo-Zero process. NGS was performed on the MiSeq Illumina platform using MiSeq reagent kit v3 (150 cycles). The RNA sequencing reads were mapped to the human genome assembly GRCh38 and the BoDV genome using the Gencode v24 annotation and TopHat v2.0.13.
Amplicon sequencing and data processing.
The amplicon sequences of the N transcripts from acutely He/80/Fct-infected OL, persistently huP2Br-infected OL, and persistently He/80/Fct-infected 293T cells were analyzed by NGS. Total RNA was extracted using TRIzol reagent, and the cDNA was synthesized using the oligo(dT) primer and the Verso cDNA synthesis kit. The cDNA of the N transcripts was amplified by PCR using the PrimeSTAR GXL DNA polymerase kit (TaKaRa). Amplicon libraries were prepared using the Kapa Hyper Prep kit. Deep sequencing was performed on the MiSeq Illumina platform using MiSeq reagent kit v3 (150 cycles). The RNA sequencing reads were mapped to the N transcript sequence using TopHat.
Molecular dynamics simulations.
The initial coordinates of the N1 tetramer were taken from the cocrystal structure (PDB accession number 1N93) (32). The missing loops at positions 315 to 322 in each monomer were added using Molecular Operating Environment (MOE) software (version 2018; Chemical Computing Group). The structural model of the N3 tetramer was constructed in silico using the N1 tetramer structure. After deleting amino acid residues 179 to 218 of the N1 tetramer in each monomer, the Loop Modeler module of MOE was used to create a bond between amino acids 178 and 219 (N1 numbering) in N3. Protonation states of the ionizable residues were assigned at pH 7.0 using the PDB2PQR Web server (51). All missing hydrogen atoms were added with the LEaP module in AMBER 16 (Conflex USA) (52). The ff14SB force field was applied for N1 and N3 tetramers (53). The total charges of the proteins were neutralized by the addition of chloride counterions. The systems were then solvated in a truncated octahedral box of transferable intermolecular potential with 3 points (TIP3P) water molecules with a distance of at least 10 Å around the protein. All energy minimization and molecular dynamics (MD) simulations were performed using the pmemd.cuda program in AMBER 16, with a cutoff radius of 10 Å for the nonbonded interactions. The locations of hydrogen atoms, water molecules, and counterions were optimized to remove bad contacts. The energy of each system was then minimized without any constraints using the steepest-descent method for 500 steps, followed by the conjugate gradient method for 1,500 steps. After minimization of energy, the system was gradually heated from 0 K to 310 K over 300 ps with harmonic restraints (with a force constant of 1.0 kcal/mol · Å2). Two additional rounds of MD (50 ps each at 310 K) were performed with a decreasing restraint weight reduced from 0.5 to 0.1 kcal/mol · Å2. Next, 1.0 μs of an unrestrained production run was performed, and the production trajectories were collected every 10 ps. All MD simulations were performed using the NPT ensemble and the Berendsen algorithm to control temperature and pressure (54). The time step was 2 fs, and the SHAKE algorithm was used to constrain all bond lengths involving hydrogen atoms (55). Long-range electrostatic interactions were treated using the particle mesh Ewald method (56).
The stability of the trajectories was assessed by monitoring the root mean square deviations (RMSDs) of the backbone heavy atoms in comparison with the initial structure. After confirming that RMSDs in all systems reached equilibrium within 500 ns, the trajectories were extracted from the simulations from 500 to 1,000 ns in the analyses.
Data availability.
The sequences reported in this article have been deposited in the DNA Data Bank of Japan (DDBJ) under Sequence Read Archive accession numbers DRA007567 (total mRNA sequences of uninfected and BoDV-infected OL cells) and DRA007646 (amplicon sequences of BoDV N mRNA).
ACKNOWLEDGMENTS
This study was supported in part by JSPS KAKENHI grants JP17H04083 (K.T.); MEXT KAKENHI grants JP16H06429, JP16K21723, and JP16H06430 (K.T.); JSPS Core-to-Core Program A; the Advanced Research Networks (K.T.); and AMED grants JP18am0301015 and JP18fm020814 (K.T.).
REFERENCES
- 1.Wasilewski M, Chojnacka K, Chacinska A. 2017. Protein trafficking at the crossroads to mitochondria. Biochim Biophys Acta Mol Cell Res 1864:125–137. doi: 10.1016/j.bbamcr.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Ullman KS, Powers MA, Forbes DJ. 1997. Nuclear export receptors: from importin to exportin. Cell 90:967–970. doi: 10.1016/S0092-8674(00)80361-X. [DOI] [PubMed] [Google Scholar]
- 3.von Heijne G. 1990. The signal peptide. J Membr Biol 115:195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]
- 4.Hauri H-P, Schweizer A. 1992. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol 4:600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson RG, Lamb RA. 1990. RNA editing by G-nucleotide insertion in mumps virus P-gene mRNA transcripts. J Virol 64:4137–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firth AE, Brierley I. 2012. Non-canonical translation in RNA viruses. J Gen Virol 93:1385–1409. doi: 10.1099/vir.0.042499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenik M, Chebli K, Blondel D. 1995. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J Virol 69:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata K, Hayashibara T, Sugahara K, Uemura A, Yamaguchi T, Harasawa H, Hasegawa H, Tsuruda K, Okazaki T, Koji T, Miyanishi T, Yamada Y, Kamihira S. 2006. A novel alternative splicing isoform of human T-cell leukemia virus type 1 bZIP factor (HBZ-SI) targets distinct subnuclear localization. J Virol 80:2495–2505. doi: 10.1128/JVI.80.5.2495-2505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulshreshtha V, Ayalew LE, Islam A, Tikoo SK. 2014. Conserved arginines of bovine adenovirus-3 33K protein are important for transportin-3 mediated transport and virus replication. PLoS One 9:e101216. doi: 10.1371/journal.pone.0101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briese T, Schneemann A, Lewis AJ, Park YS, Kim S, Ludwig H, Lipkin WI. 1994. Genomic organization of Borna disease virus. Proc Natl Acad Sci U S A 91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwemmle M, Jehle C, Shoemaker T, Lipkin WI. 1999. Characterization of the major nuclear localization signal of the Borna disease virus phosphoprotein. J Gen Virol 80:97–100. doi: 10.1099/0022-1317-80-1-97. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Shoya Y, Koda T, Takashima I, Lai PK, Ikuta K, Kakinuma M, Kishi M. 1998. Nuclear targeting activity associated with the amino terminal region of the Borna disease virus nucleoprotein. Virology 243:188–197. doi: 10.1006/viro.1998.9049. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Kamitani W, Zhang G, Watanabe M, Tomonaga K, Ikuta K. 2001. Borna disease virus nucleoprotein requires both nuclear localization and export activities for viral nucleocytoplasmic shuttling. J Virol 75:3404–3412. doi: 10.1128/JVI.75.7.3404-3412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker MP, Lipkin WI. 2002. Characterization of the nuclear localization signal of the Borna disease virus polymerase. J Virol 76:8460–8467. doi: 10.1128/JVI.76.16.8460-8467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanai H, Kobayashi T, Hayashi Y, Watanabe Y, Ohtaki N, Zhang G, de la Torre JC, Ikuta K, Tomonaga K. 2006. A methionine-rich domain mediates CRM1-dependent nuclear export activity of Borna disease virus phosphoprotein. J Virol 80:1121–1129. doi: 10.1128/JVI.80.3.1121-1129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff T, Unterstab G, Heins G, Richt JA, Kann M. 2002. Characterization of an unusual importin alpha binding motif in the Borna disease virus p10 protein that directs nuclear import. J Biol Chem 277:12151–12157. doi: 10.1074/jbc.M109103200. [DOI] [PubMed] [Google Scholar]
- 17.Pyper JM, Gartner AE. 1997. Molecular basis for the differential subcellular localization of the 38- and 39-kilodalton structural proteins of Borna disease virus. J Virol 71:5133–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoya Y, Kobayashi T, Koda T, Ikuta K, Kakinuma M, Kishi M. 1998. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J Virol 72:9755–9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Zhang G, Lee B-J, Baba S, Yamashita M, Kamitani W, Yanai H, Tomonaga K, Ikuta K. 2003. Modulation of Borna disease virus phosphoprotein nuclear localization by the viral protein X encoded in the overlapping open reading frame. J Virol 77:8099–8107. doi: 10.1128/JVI.77.14.8099-8107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg M, Ehrenborg C, Blomberg J, Pipkorn R, Berg AL. 1998. Two domains of the Borna disease virus p40 protein are required for interaction with the p23 protein. J Gen Virol 79:2957–2963. doi: 10.1099/0022-1317-79-12-2957. [DOI] [PubMed] [Google Scholar]
- 21.Schneider U, Naegele M, Staeheli P. 2004. Regulation of the Borna disease virus polymerase complex by the viral nucleoprotein p38 isoform. Arch Virol 149:1409–1414. doi: 10.1007/s00705-004-0327-6. [DOI] [PubMed] [Google Scholar]
- 22.Perez M, Sanchez A, Cubitt B, Rosario D, de la Torre JC. 2003. A reverse genetics system for Borna disease virus. J Gen Virol 84:3099–3104. doi: 10.1099/vir.0.19467-0. [DOI] [PubMed] [Google Scholar]
- 23.Schneider PA, Schneemann T, Lipkin WI. 1994. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J Virol 68:5007–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cubitt B, Oldstone C, Valcarcel J, de la Torre JC. 1994. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res 34:69–79. doi: 10.1016/0168-1702(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 25.Tomonaga K, Kobayashi T, Lee BJ, Watanabe M, Kamitani W, Ikuta K. 2000. Identification of alternative splicing and negative splicing activity of a nonsegmented negative-strand RNA virus, Borna disease virus. Proc Natl Acad Sci U S A 97:12788–12793. doi: 10.1073/pnas.97.23.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz Y, Wang ET, Airoldi EM, Burge CB. 2010. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods 7:1009–1015. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneemann A, Schneider PA, Kim S, Lipkin WI. 1994. Identification of signal sequences that control transcription of Borna disease virus, a nonsegmented, negative-strand RNA virus. J Virol 68:6514–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Hayashi Y, Omori H, Honda T, Daito T, Horie M, Ikuta K, Fujino K, Nakamura S, Schneider U, Chase G, Yoshimori T, Schwemmle M, Tomonaga K. 2012. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe 11:492–503. doi: 10.1016/j.chom.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Sonnhammer ELL, Von Heijne G, Krogh A. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol 6:175–182. [PubMed] [Google Scholar]
- 30.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 31.Cui J, Chen W, Sun J, Guo H, Madley R, Xiong Y, Pan X, Wang H, Tai AW, Weiss MA, Arvan P, Liu M. 2015. Competitive inhibition of the endoplasmic reticulum signal peptidase by non-cleavable mutant preprotein cargos. J Biol Chem 290:28131–28140. doi: 10.1074/jbc.M115.692350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph MG, Kraus I, Dickmanns A, Eickmann M, Garten W, Ficner R. 2003. Crystal structure of the Borna disease virus nucleoprotein. Structure 11:1219–1226. doi: 10.1016/j.str.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Fujino K, Horie M, Honda T, Merriman DK, Tomonaga K. 2014. Inhibition of Borna disease virus replication by an endogenous bornavirus-like element in the ground squirrel genome. Proc Natl Acad Sci U S A 111:13175–13180. doi: 10.1073/pnas.1407046111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, Tomonaga K. 2010. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 463:84–87. doi: 10.1038/nature08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukai Y, Horie M, Tomonaga K. 2018. Systematic estimation of insertion dates of endogenous bornavirus-like elements in vesper bats. J Vet Med Sci 80:1356–1363. doi: 10.1292/jvms.18-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siemetzki U, Ashok MS, Briese T, Lipkin WI. 2009. Identification of RNA instability elements in Borna disease virus. Virus Res 144:27–34. doi: 10.1016/j.virusres.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubois J, Terrier O, Rosa-Calatrava M. 2014. Influenza viruses and mRNA splicing: doing more with less. mBio 5:e00070-14. doi: 10.1128/mBio.00070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua MA, Schmid S, Perez JT, Langlois RA, tenOever BR. 2013. Influenza A virus utilizes suboptimal splicing to coordinate the timing of infection. Cell Rep 3:23–29. doi: 10.1016/j.celrep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzoor R, Igarashi M, Takada A. 2017. Influenza A virus M2 protein: roles from ingress to egress. Int J Mol Sci 18:E2469. doi: 10.3390/ijms18122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuwata R, Isawa H, Hoshino K, Tsuda Y, Yanase T, Sasaki T, Kobayashi M, Sawabe K. 2011. RNA splicing in a new rhabdovirus from Culex mosquitoes. J Virol 85:6185–6196. doi: 10.1128/JVI.00040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillon PJ, Gupta KC. 1989. Expression of five proteins from the Sendai virus P/C mRNA in infected cells. J Virol 63:974–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song W, Kao W, Zhai A, Qian J, Li Y, Zhang Q, Zhao H, Hu Y, Li H, Zhang F. 2013. Borna disease virus nucleoprotein inhibits type I interferon induction through the interferon regulatory factor 7 pathway. Biochem Biophys Res Commun 438:619–623. doi: 10.1016/j.bbrc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Schneider U, Blechschmidt K, Schwemmle M, Staeheli P. 2004. Overlap of interaction domains indicates a central role of the P protein in assembly and regulation of the Borna disease virus polymerase complex. J Biol Chem 279:55290–55296. doi: 10.1074/jbc.M408913200. [DOI] [PubMed] [Google Scholar]
- 44.Hock M, Kraus I, Schoehn G, Jamin M, Andrei-Selmer C, Garten W, Weissenhorn W. 2010. RNA induced polymerization of the Borna disease virus nucleoprotein. Virology 397:64–72. doi: 10.1016/j.virol.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Makino A, Fujino K, Parrish NF, Honda T, Tomonaga K. 2015. Borna disease virus possesses an NF-κB inhibitory sequence in the nucleoprotein gene. Sci Rep 5:8696. doi: 10.1038/srep08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura Y, Takahashi H, Shoya Y, Nakaya T, Watanabe M, Tomonaga K, Iwahashi K, Ameno K, Momiyama N, Taniyama H, Sata T, Kurata T, de la Torre JC, Ikuta K. 2000. Isolation of Borna disease virus from human brain tissue. J Virol 74:4601–4611. doi: 10.1128/JVI.74.10.4601-4611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujino K, Yamamoto Y, Daito T, Makino A, Honda T, Tomonaga K. 2017. Generation of a non-transmissive Borna disease virus vector lacking both matrix and glycoprotein genes. Microbiol Immunol 61:380–386. doi: 10.1111/1348-0421.12505. [DOI] [PubMed] [Google Scholar]
- 48.Daito T, Fujino K, Honda T, Matsumoto Y, Watanabe Y, Tomonaga K. 2011. A novel Borna disease virus vector system that stably expresses foreign proteins from an intercistronic noncoding region. J Virol 85:12170–12178. doi: 10.1128/JVI.05554-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kojima S, Honda T, Matsumoto Y, Tomonaga K. 2014. Heat stress is a potent stimulus for enhancing rescue efficiency of recombinant Borna disease virus. Microbiol Immunol 58:636–642. doi: 10.1111/1348-0421.12193. [DOI] [PubMed] [Google Scholar]
- 50.Yanai H, Hayashi Y, Watanabe Y, Ohtaki N, Kobayashi T, Nozaki Y, Ikuta K, Tomonaga K. 2006. Development of a novel Borna disease virus reverse genetics system using RNA polymerase II promoter and SV40 nuclear import signal. Microbes Infect 8:1522–1529. doi: 10.1016/j.micinf.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA. 2007. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res 35:W522–W525. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Case DA, Betz RM, Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N, Izadi S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Lin C, Luchko T, Luo R, Madej B, Mermelstein D, Merz KM, Monard G, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg A, Sagui C, Simmerling CL, Botello-Smith WM, Swails J, Walker RC, Wang J, Wolf RM, Wu X, Xiao L, Kollman PA. 2016. AMBER 2016. University of California, San Francisco, San Francisco, CA. [Google Scholar]
- 53.Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C. 2015. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713. doi: 10.1021/acs.jctc.5b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berendsen HJC, Postma JPM, Van Gunsteren WF, Dinola A, Haak JR. 1984. Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690. doi: 10.1063/1.448118. [DOI] [Google Scholar]
- 55.Ryckaert JP, Ciccotti G, Berendsen HJC. 1977. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 56.Darden T, York D, Pedersen L. 1993. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092. doi: 10.1063/1.464397. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences reported in this article have been deposited in the DNA Data Bank of Japan (DDBJ) under Sequence Read Archive accession numbers DRA007567 (total mRNA sequences of uninfected and BoDV-infected OL cells) and DRA007646 (amplicon sequences of BoDV N mRNA).