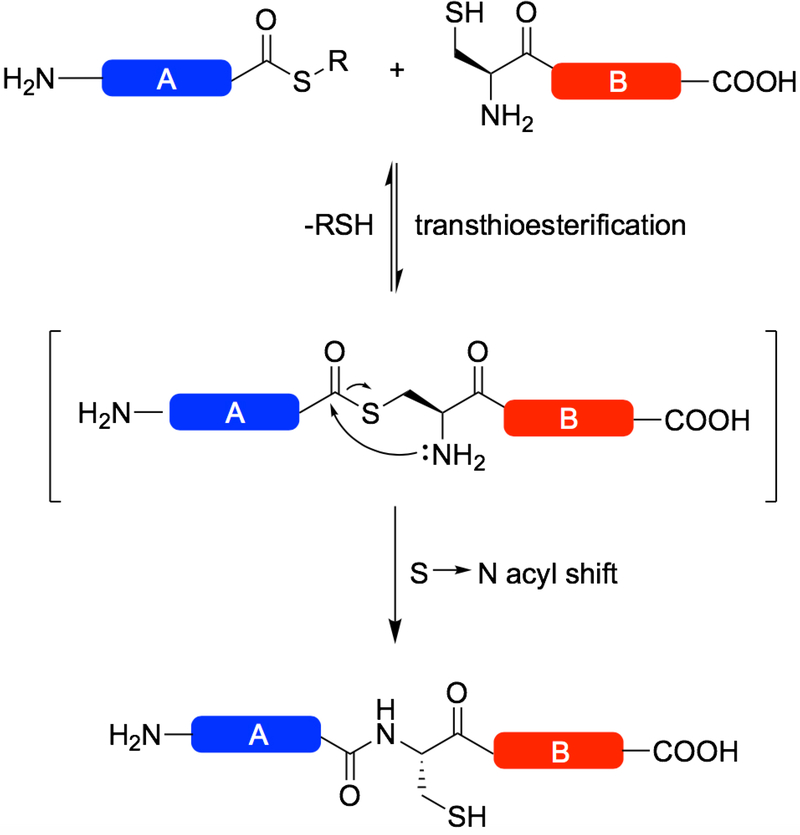
Figure 1:
Scheme describing Native Chemical Ligation (NCL). An unprotected peptide fragment bearing an N-terminal Cys residue attacks the C-terminal thioester of another unprotected peptide fragment, undergoing transthioesterification and subsequent S to N acyl transfer to form an amide bond at the ligation site.