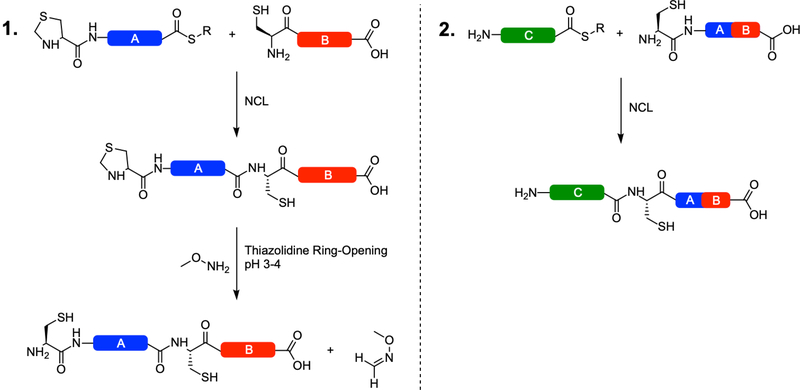
Figure 2:
Thiazolidine decryption via methoxyamine at the N-terminus of a ligated peptide and subsequent sequential ligation(s). Following the first NCL reaction, the thiazolidine ring is opened in acidic conditions, freeing formaldehyde (Panel 1). The formaldehyde must be scavenged by a methoxy compound to prevent ring reformation. The resulting fragment AB is then purified by RP-HPLC and lyophilized for use as the C-terminal fragment in the next NCL reaction (Panel 2). In this manner, the protein is constructed in the C-terminal to N-terminal direction. Note that Fragment C could also function as an intermediate fragment, bearing a thiazolidine group at its N-terminus and be ring-opened in the same manner as in Panel 1. This approach is repeated for as many fragments as necessary to complete the protein.