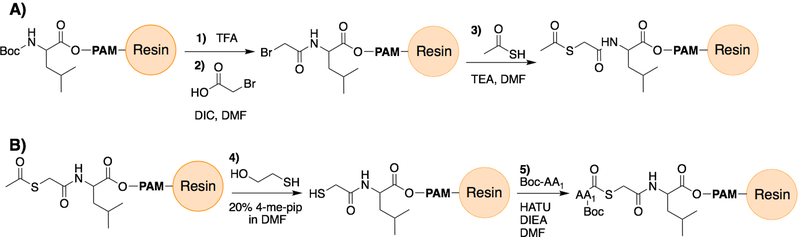
Figure 3:
Scheme describing the on-resin assembly of the mercaptoacetic acid leucine thioester (MAAL) moiety for use in Boc-SPPS. Panel A shows (1) Boc deprotection by TFA and (2) diisopropylcarbodiamide (DIC)-promoted acylation by bromoacetic acid in DMF, followed by (3) displacement through treatment with thioacetic acid in triethylamine (TEA). Panel B described the (4) unmasking of the thioacetic thioester to a free sulfhydryl group by treatment with 2-mercaptoethanol in 20% 4-methylpiperidine solution, which is then (5) acetylated with the C-terminal Boc-protected amino acid of the desired peptide fragment by routine SPPS coupling conditions (HATU, DIEA, DMF).