Figure 2.
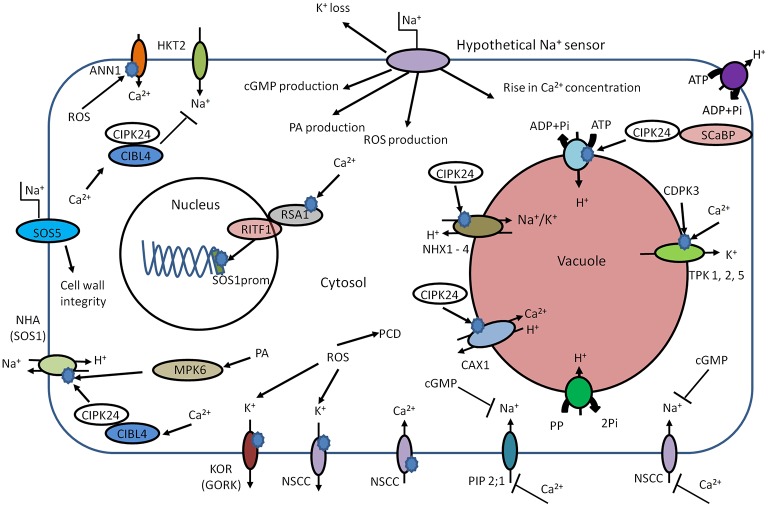
Schematic overview of early components involved in salt sensing. High external Na+ concentration leads to elevation of intracellular Ca2+, phosphatidic acid (PA), and cGMP. Cld PA can activate NHA (SOS1) in an independent manner. The main target of Ca2+ is CIBL4 (SOS3). The CIBL4 is capable to form the complex with CBL-interacting serine/threonine-protein kinase 24 (CIPK24, SOS2). The CIBL4-CIPK24 complex activates NHA (SOS1) and inhibits Na+ uptake by HKT2. CIPK24 together with SCaBP (SOS3 like protein) is involved in activation of the V-ATPase. CIPK24 participates in the activation of vacuolar transporters such as CAX and NHX. The rise in cytosolic Ca2+ concentration could trigger interaction of RSA1-RITF1. RSA1-RITF1 complex activates promoter of SOS1 gene. PA is involved in activation of mitogen-activated protein kinase 6 (MPK6). MPK6 can directly phosphorylate SOS1. The Ca2+-dependent kinase (CDPK3) and cytosolic Ca2+ lead to activation of vacuolar two-pore K+ channels (TPKs) and subsequent K+ release from vacuole. Due to the plasma membrane localization, SOS5 protein is considered to be potential candidate for extracellular Na+ sensing and helps maintain of cell wall integrity and architecture. Annexin1 (ANN1) is capable to sense the high concentrations of extracellular Na+ by mediating ROS-activated Ca2+ influx through the plasma membrane of plant cells. The rise of cGMP leads to inhibition of Na+ uptake, possibly via cyclic nucleotide–gated ion channels (CNGSs) and glutamate receptor (GLRs). PIP2;1, CNGCs, and GLRs could be blocked by exogenous Ca2+. The ROS production leads to K+ leak via activation of outward K+ channels – KOR (guard cells outward K+ channel, GORK) and NSCC. The intracellular accumulation of ROS at high levels can trigger programmed cell death (PCD).