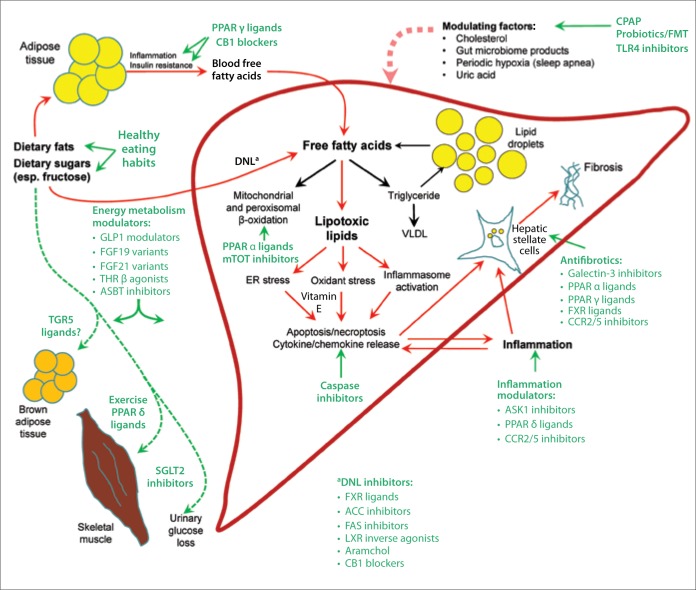
Figure.
The substrate overload lipotoxic liver injury model illustrates the pathogenesis of nonalcoholic steatohepatitis (NASH) and targets of therapy. Based on extensive cell culture, animal studies, and human trials, free fatty acids play a central role in the pathogenesis of NASH. Free fatty acids can come from lipolysis of triglyceride in adipose tissue, and are delivered to the liver through the blood. A significant contributor to the free fatty acid flow through the liver is the process of de novo lipogenesis (DNL), whereby hepatocytes synthesize new fatty acids from excess carbohydrates, especially fructose. Fatty acids in hepatocytes can be metabolized by mitochondrial and peroxisomal β-oxidation and converted into triglycerides. Triglycerides can then either be excreted into the blood as very-low-density lipoprotein (VLDL) or stored in lipid droplets. Lipid droplet triglyceride undergoes regulated lipolysis to release fatty acids back into the hepatocyte–free fatty acid pool. When the disposal of fatty acids through β-oxidation or the formation of triglyceride is impaired or overwhelmed by substrate overload, fatty acids can be converted into a number of lipotoxic species that lead to endoplasmic reticulum (ER) stress, oxidant stress, and inflammasome activation. These processes cause the cell injury, inflammation, stellate cell activation, and progressive accumulation of excess extracellular matrix that characterize NASH. Lifestyle modifications focused on healthy eating habits and regular exercise reduce the substrate overload by decreasing intake and diverting energy substrates to metabolically active tissues such as skeletal muscle, thus preventing or reversing NASH. A number of pharmacotherapies are currently being evaluated in clinical trials and are shown with their primary targets. DNL is a target of many therapies by downregulation of the enzymes of DNL (eg, ACC inhibition) or reduction of the expressions of the enzymes of DNL (eg, FXR ligands).
ACC, acetyl coenzyme A carboxylase; ASBT, apical sodium-dependent bile acid transporter; ASK1, apoptosis signal-regulating kinase 1; CB1, cannabinoid receptor 1; CCR, C-C motif chemokine receptor; CPAP, continuous positive airway pressure; FAS, fatty acid synthetase; FGF, fibroblast growth factor; FMT, fecal microbiota transplant; FXR, farnesoid X receptor; GLP1, glucagon-like peptide-1; LXR, liver X receptor; mTOT, mitochondrial target of thiazolidinediones; PPAR, peroxisome proliferator-activated receptor; SGLT2, sodium-glucose cotransporter 2; TGR5, Takeda G-protein coupled receptor-5; THR, thyroid hormone receptor; TLR4, toll-like receptor 4.