Figure 2.
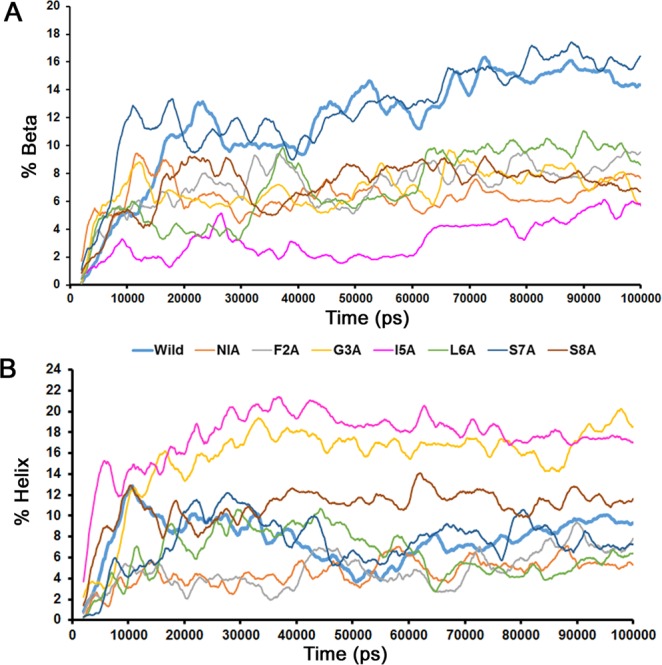
Secondary structural transition observed for all the systems within 100 ns. (A) Time dependence of β-structure. Maximum (14–16%) β-structure was observed for Wild-type and S7A peptides and minimum (>6%) was observed for I5A peptide oligomers. (B) Time dependence of helical structure formation. Maximum (16–20%) helical content was observed for G3A and I5A, while for all other analogs the content was less than 12%. The percent of helix and β-sheet was calculated using DSSP.
