Fig. 1.
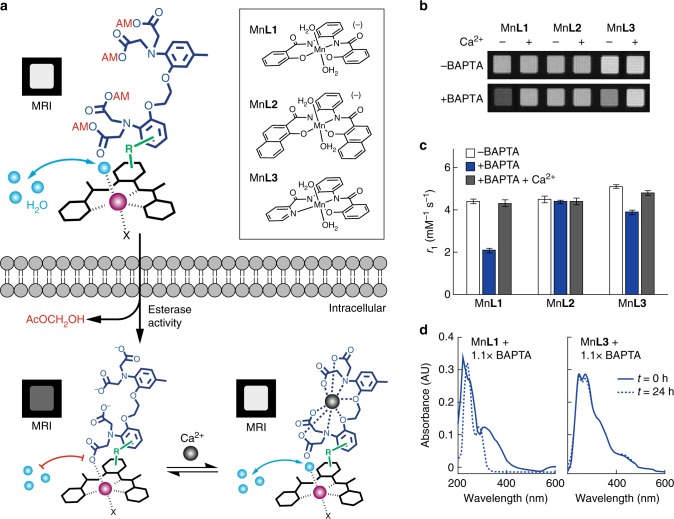
Design of cell permeable sensors for calcium-dependent molecular fMRI. a The design consists of a cell permeable paramagnetic platform (black complex, Mn-PDA candidate complexes shown at top right), a BAPTA-based calcium chelator (dark blue), and a linker connecting them (green, R). Prior to cell entry (top) the BAPTA carboxylates are protected with cleavable AM esters (red), and water exchange (cyan spheres) is expected to take place at the paramagnetic metal center (purple), leading to T1-weighted MRI signal enhancement (labeled MRI). When the agent enters cells (bottom left), the AM esters are cleaved, liberating the sensor in its calcium-free “off” state; in this state, water exchange is expected to be blocked by interactions between the BAPTA moiety and Mn3+, leading to low MRI signal. When calcium binds (bottom right), the MRI signal could increase again as interactions between the BAPTA and paramagnetic platform are reduced. b Evaluation of interactions between untethered BAPTA and Mn-PDA contrast agents. In the absence of BAPTA, the Mn3+ complexes (40 µM) do not produce calcium-dependent T1-weighted MRI contrast changes (top), but in the presence of 1.1 equiv BAPTA, both MnL1 and MnL3 display sensitivity to addition of 1 mM calcium (bottom). c Longitudinal relaxivity (r1) values corresponding to the conditions in panel b. Error bars denote SD of four measurements. d Optical spectra of contrast agents MnL1 and MnL3 in the presence of BAPTA after incubation for 0 h (solid) or 24 h (dashed), indicating comparative stability of MnL3