Abstract
Background
In Asia, serotype K1/K2 Klebsiella pneumoniae are the major capsular serotypes that cause liver abscess or bacteremia in patients. The purpose of this study was to compare novel immunochromatographic strips (ICSs), which can rapidly detect K. pneumoniae serotypes K1/K2 in clinical samples, to conventional capsular serotyping methods.
Methods
Pus drainage samples from 16 patients with a liver abscess caused by K. pneumoniae, blood samples from 112 positive flagged blood culture bottle and a subsequent single colony in the medium were tested with the ICS. The results were then compared to findings of capsular swelling tests. Samples subjected to the polymerase chain reaction (PCR) analysis were used as reference.
Results
The identification of K. pneumoniae via the traditional bacterial culture from pus samples took 3.4 days on average (ranging from 2.2 to 5.5 days). Further capsular serotyping of K. pneumoniae by the capsular swelling test of pure isolates lasted 5–10 min, and the PCR method took ~ 4 h. As for ICSs, the time for direct identification of the K. pneumoniae capsular serotype K1/K2 in pus was < 4 min (ranging from 2 to 4 min). The results of ICSs were consistent with capsular swelling tests and PCR methods. Testing of 112 blood culture samples and subsequent single colonies in the medium with ICSs yielded consistent results for most samples.
Conclusions
This study indicates that ICSs can rapidly detect K. pneumoniae serotypes K1 and K2 in pus or positive flagged blood culture broth samples within 5 min. Their accuracy is comparable to that of the conventional capsular serotyping methods such as a serum agglutination assay or PCR.
Keywords: Klebsiella pneumoniae, Serotypes K1, Serotype K2, Serum agglutination, PCR, ICS
Background
Pyogenic liver abscesses caused by Klebsiella pneumoniae constitute an emerging global infectious disease as reported recently [1]. Among 79 serotypes of the capsular polysaccharides of K. pneumoniae, serotypes K1/K2 have the highest prevalence in many southeast Asian countries and are associated with virulent types in K. pneumoniae-induced bacteremia and liver abscesses, particularly in cases with metastatic endophthalmitis [2–4]. Appropriate early antibiotic treatment—with the aim of decreasing mortality and morbidity related to pyogenic liver abscesses caused by K. pneumoniae—is an important clinical task. It usually takes at least 3 days to isolate K. pneumoniae from pus and identify it via blood cultures in the traditional clinical laboratory setting, and serotyping is not routinely performed. The capsular swelling test via anti-capsular type sera, countercurrent immunoelectrophoresis from pure isolates, or polymerase chain reactions (PCRs) have been used to identify K. pneumoniae capsular serotypes. They take hours to days when bacterial cultures or direct clinical samples are used [5, 6]. Although the direct detection of K. pneumoniae capsular serotypes in clinical samples is possibly based on the PCR assay, false negatives may sometimes occur due to interference from a non purified DNA samples. Preparation of purified DNA may be needed to increase the accuracy of detection in a PCR assay.
In a previous study, we have evaluated a colloidal-gold-based immunochromatographic strip (ICS) kit for the rapid detection of K. pneumoniae serotypes K1 and K2 [7]. In the current study, we aimed to test the efficacy of this kit for detecting K. pneumoniae capsular serotypes directly in pus drainage samples, positive blood culture samples, and pure bacterial colonies.
Results
Comparison of different testing results on the capsular serotyping of K1 and K2 of K. pneumoniae isolates
A total of 16 patients with a liver abscess caused by K. pneumoniae, as confirmed by radiographic and microbiological analyses, were enrolled in this study. The demographic characteristics (age, gender, comorbid diabetes, duration of symptoms/signs, lobes and size of a liver abscess, duration of identification by PCR or strip test, and serotypes) of the patients are shown in Table 1.
Table 1.
Demographic data and capsular serotype identification of enrolled patients
| Case | Sex/age | DM | Duration of S/S (days) | Lobe of abscess | Size of abscess (cm) |
Identification time: | Serotypes confirmed by: | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Culture (days) | ICS (minutes) | Colony capsular swelling test |
Colony PCR | Pus ICS |
||||||
| 1 | F/56 | Yes | 5 | Left | 5.5 | 3.1 | 4 | K55 | Non-K1/K2 | Non-K1/K2 |
| 2 | F/62 | Yes | 6 | Right | 6 | 5 | 3 | K7 | Non-K1/K2 | Non-K1/K2 |
| 3 | F/65 | No | 3 | Left | 5.1 | 2.5 | 3 | K1 | K1 | K1 |
| 4 | F/45 | No | 1 | Left | 4.4 | 3.5 | 3 | K2 | K2 | K2 |
| 5 | F/62 | No | 5 | Right | 8.6 | 2.6 | 3 | K1 | K1 | K1a |
| 6 | M/41 | Yes | 9 | Right | 9.6 | 4.2 | 3 | K1 | K1 | K1 |
| 7 | M/60 | No | 5 | Right | 5.5 | 4 | 3 | K2 | K2 | K2 |
| 8 | M/51 | No | 6 | Right | 6.3 | 2.2 | 3 | K2 | K2 | K2 |
| 9 | M/88 | No | 5 | Right | 9.8 | 3.7 | 4 | K53 | Non-K1/K2 | Non-K1/K2 |
| 10 | M/42 | Yes | 5 | Right | 5 | 4.2 | 2 | K14 | Non-K1/K2 | Non-K1/K2 |
| 11 | F/84 | Yes | 3 | Right | 4.2 | 3.9 | 4 | K1 | K1 | K1a |
| 12 | F/74 | Yes | 2 | Right | 2 | 5.5 | 4 | K57 | Non-K1/K2 | Non-K1/K2 |
| 13 | M/61 | No | 2 | Right | 7.7 | 2.8 | 2 | K1 | K1 | K1 |
| 14 | M/74 | Yes | 7 | Right | 7.4 | 2.4 | 3 | K1 | K1 | K1 |
| 15 | M/61 | Yes | 14 | Right | 7.1 | 2.8 | 3 | K1 | K1 | K1 |
| 16 | M/29 | No | 5 | Right | 6 | 2.5 | 3 | K1 | K1 | K1 |
M male, F female, DM diabetes mellitus, S/S symptoms and signs, ICS immunochromatographic strip, PCR polymerase chain reaction
aEndophthalmitis was found after being confirmed to have serotype K1 by the strip test
There were nine males (56.3%, 9/16) and seven females (43.7%, 7/16) among the 16 patients with K. pneumoniae liver abscesses. Comorbid diabetes mellitus was present in 50.0% of the patients (8/16). The average diagnostic time from the start of the symptoms/signs of the liver abscess in clinical settings was 5.2 days (ranging from 1 to 14 days). The most common lobe with a liver abscess was on the right side (~ 81.3% or 13/16). The average size of a liver abscess was 6.26 cm in diameter (ranging from 2 to 9.8 cm). The duration of K. pneumoniae identification using the traditional bacteria culture for pus was 3.4 days (ranging from 2.2 to 5.5 days). Starting from a culture colony, the duration of identification of K. pneumoniae capsular serotypes by the capsular swelling test and PCR was demonstrated to require an additional 5–10 min and 4 h, respectively. For the rapid strip test, the time for identification of K. pneumoniae capsular serotype K1/K2 directly in pus was only 3.1 min (ranging from 2 to 4 min).
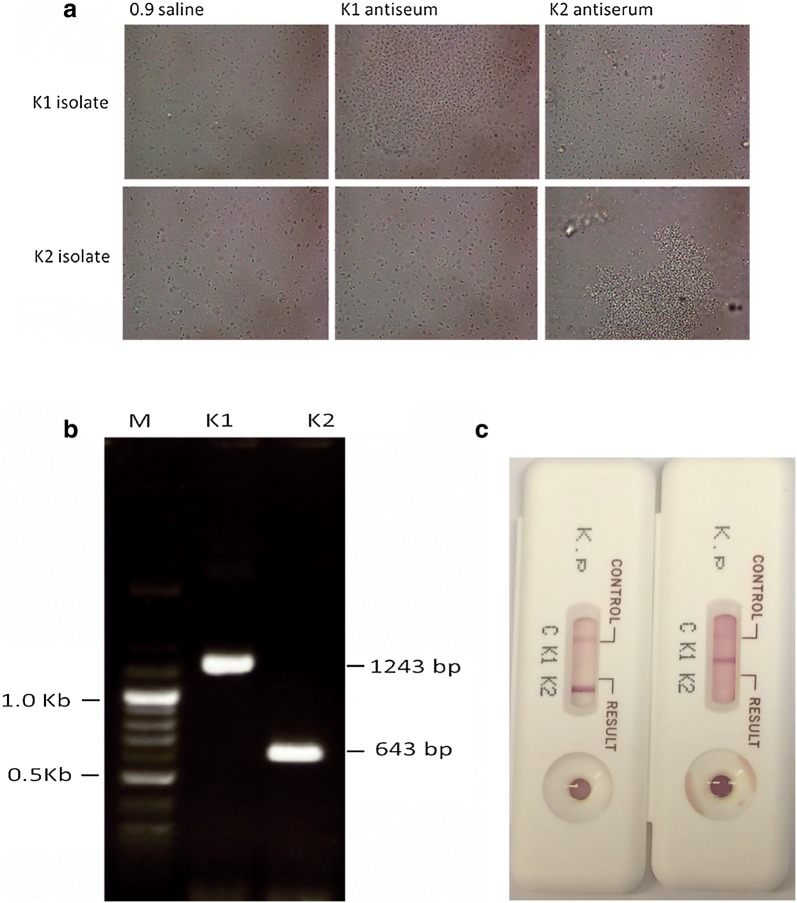
Compared to the PCR method, which served as a reference, both the capsular swelling and strip tests yielded consistent results on identification of serotypes K1 and K2. Results on serotypes K1 and K2 from different methods are presented in Fig. 1a–c. Among the 16 K. pneumoniae isolates from liver abscesses, 50.0% (8/16) turned out to have serotype K1, 18.8% (3/16) serotype K2, and 31.3% (5/16) non-K1/K2 serotype. The five K. pneumoniae non-K1/K2 serotypes were K55, K7, K53, K14, and K57 as confirmed by the capsular swelling test. In eight patients with K1 K. pneumoniae liver abscesses, two patients with complications of distant metastatic endophthalmitis according to clinical findings (posterior or anterior ocular inflammation via funduscopic examination) and radiological studies. Sixteen strains from patients with K. pneumoniae liver abscesses were analyzed via pulse-field gel electrophoresis (PFGE). Most strains had distinct PFGE patterns except for the isolate number 3 and 5, which were clonally indistinguishable (Fig. 2).
Fig. 1.
The results of serotypes K1 and K2 K. pneumoniae identification by different typing methods. a Capsular swelling test showing capsular serotypes K1/K2 following reaction with K1 and K2 antiserum. Saline (0.9%) was used as control. Images were captured using a phase contrast microscope (original magnification, 1000×). b Capsular serotypes K1/K2 detected by the PCR testing. Visualization of the bands on an agarose (1.5%) gel for the capsular serotypes K1/K2 strains (K1, K2). Size of each amplicon is indicated at the side. M, markers. c Capsular serotypes K1/K2 detected by means of ICSs. The red line in the ICS indicated serotype K1 or K2 is shown
Fig. 2.
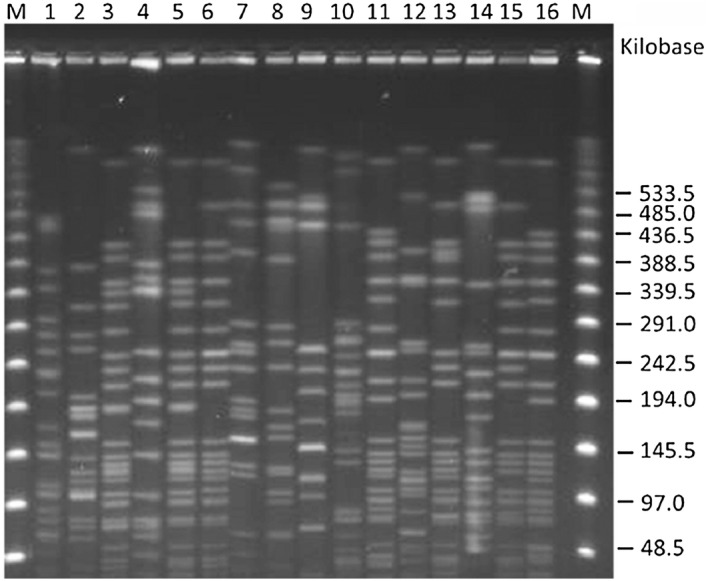
Pulsed-field gel electrophoresis (PFGE) analysis of K. pneumoniae strains isolated from 16 enrolled patients. Banding patterns determined by PFGE of 16 isolates of K. pneumoniae were divergent except for the isolate number 3 and 5, which were clonally indistinguishable
ICS analysis of positive flagged blood culture broth samples and pure bacterial culture
A total of 112 blood culture broth samples with positive signals for microorganisms and subsequent pure bacterial colony growth on solid culture media were included in ICS efficacy testing. Among the 108 K. pneumoniae samples, testing of blood culture broth samples with ICS revealed that 14 belonged to serotype K1 and 16 belonged to serotype K2. Testing for a single colony with ICS produced consistent results for most samples. Different results between tests of blood culture broth samples and tests of a single colony were obtained, indicating that two non-K1/K2 results from blood broth samples were identified as the K2 serotype in the pure single colony. Subsequent PCR typing of the two isolates with discrepant results revealed that all of them were of the K5 serotype. For the four non-K. pneumoniae samples from blood culture broth samples and pure bacterial culture (Staphylococcus capitis, S. aureus, Proteus mirabilis, and Pseudomonas aeruginosa), negative results were produced by the ICS kit (Table 2).
Table 2.
ICS test results on 108 K. pneumoniae isolates from blood culture bottles and pure colonies
| Results of strip | Blood culture broth | Single colony | ||||
|---|---|---|---|---|---|---|
| K1(+) | K2(+) | Negative | K1(+) | K2(+) | Negative | |
| Case Number | 14 | 16a | 78a | 14 | 18 | 76 |
ICS immunochromatographic strip
aTwo non-K1/K2 isolates from blood culture broth samples were identified as the K2 serotype in subsequent single colony broth samples and further serotyping by PCR confirmed serotype K5
Discussion
Capsular polysaccharide has been shown to be an important virulent factor of K. pneumoniae infection pathogenicity [8]. Among the 79 recognized capsular serotypes, K1 and K2 have been reported to have a significant association with increased virulence and septicemia-related infection rates, as well as liver abscesses associated with distant metastasis, including endophthalmitis [5]. In a recent study out of a French intensive care unit, hypervirulent K. pneumoniae infections were found to result mostly from community-acquired infections and were predominately caused by K. pneumoniae serotypes K1 and K2. Although mortality rates between hypervirulent and nonhypervirulent K. pneumoniae strains were not different, these community-acquired hypervirulent K. pneumoniae strains yielded higher rates of multiorgan failure [9]. In addition, antiphagocytosis activity and serum resistance to bactericidal activity for K. pneumoniae with capsular serotype K1 and K2 antigens have been reported regarding clinical K. pneumoniae infections [4].
In vitro and in vivo studies have revealed that serotypes K1 and K2 cause an increase in disease lethality [10]. According to an epidemiological study on K. pneumoniae-related liver abscesses in endemic regions, most cases are monomicrobial infections [11–13]. Some studies have shown that the strains from community-acquired liver abscesses are highly susceptible to cefazolin and other extended-spectrum antibiotics, including cephalosporins [3, 14]. The susceptibility of K. pneumoniae in liver abscesses has remained unchanged even in recurrent K. pneumoniae liver abscesses [15, 16]. Because broad-spectrum antimicrobials combined with earlier abscess drainage for patients was recommended [15], rapid detection of the infection could help to confirm the appropriate regimen for these patients [1], resulting in a reduction in medical costs. This way, shorter hospital stay in addition to lower antibiotic consumption could be achieved. In addition, early K1 and K2 detection will alert physicians to possible distant metastatic infectious complications such as endophthalmitis. In our study, there were no deaths after the rapid identification of K1 and K2 capsular serotypes, with the administration of adequate antibiotics. Two patients with distant endophthalmitis-associated metastases with serotype K1 identified by ICSs had a good prognosis due to quick management with systemic antibiotics and local treatment.
Apart from direct pus analysis after drainage, ICS application to a positive blood culture medium yielded matching results, when compared to pure bacterial cultures. In clinical microbiology laboratories, blood culture shows positive signals for microorganisms, and the process usually takes approximately one more day for subculture formation and identification. Using the ICSs, it is possible to rapidly identify K. pneumoniae serotypes K1 and K2 in samples with a high probability of K. pneumoniae bacteremia in an endemic area, such as in liver abscess cases in Southeast Asia [17, 18]. The ICSs may be valuable for selecting the antimicrobial therapy in spite of broad antimicrobial coverage for bacteremia cases. Nonetheless, in view of the various pathogens that may be cultured in blood culture systems, the drawback of the ICS application to positive blood culture samples is a high negative rate, because ICS cannot detect all K. pneumoniae bacteremia strains, only serotypes K1 and K2. This approach should be more helpful if a new ICS kit which includes detection of additional specific K. pneumoniae strains could be developed. Although our results revealed a high concordance rate for testing of positive blood culture samples and isolates, there was a discrepant result. We suspect that the discrepancy resulted from the nutritious blood culture broth which contains many substances which might interfere with the antigen–antibody reaction.
Among all the various serotypes of K. pneumoniae used to assess the specificity of the ICS kit in previous studies, there were no false positive or false negative results produced by the ICS kit, and these data were comparable to PCR and serum agglutination results [7]. In the present study, the color reactions for pus in both the control and the test lines were seen within 3 to 4 min for both serotypes K1 and K2. Positive color reactions for the control line and negative reactions for the test line in our test were obtained for K. pneumoniae serotypes other than K1 and K2, which were later demonstrated to be serotypes K7, K14, K53, K55, and K57. PFGE findings revealed that most strains tested in the current study were unrelated sporadic isolates. After the comparison of the ICSs to other conventional test methods—PCR and serum agglutination—the results were consistent. This suggested that ICSs have high specificity, equal to that of other methods on different clinical K. pneumoniae strains..
Traditional serotyping methods include serum agglutination, countercurrent immunoelectrophoresis, and PCR, which have been the standard methods for confirming K. pneumoniae capsular serotypes [2, 4, 5, 7, 19, 20]. Nevertheless, these methods require specific materials such as larger doses of anticapsular serum, primers, chemical mixes, and gel preparations in addition to well-qualified technicians and equipment. This situation can cause delays with the results, not to mention the complicated procedure. Although PCR is a more sensitive test with a very low detection limit, it took 4 h. Unlike conventional detection methods, the rapid ICS method does not require skilled technicians or special equipment. In fact, it is extremely easy to use because it requires only simple visual analysis. Moreover, ICS specificity is comparable to that of other methods. The advantage of the ICS method described here is that ICSs do not require significant preparation before testing the pus directly after ultrasonography-guided or computed tomography (CT)-guided aspiration.
Conclusions
In summary, this study revealed that ICSs are highly specific and sensitive for the direct detection of K. pneumoniae serotypes K1 and K2 in pus and samples of positive blood culture broth in clinical settings, as compared with serum agglutination with countercurrent immunoelectrophoresis and PCR from a culture colony. ICSs can serve as a rapid tool for the identification of hyper-virulent K. pneumoniae strains that can cause a liver abscess.
Methods
Patients
The study was conducted at the Tri-Service General Hospital, a teaching hospital with 1800 beds in northern Taiwan and at Kaohsiung Medical University Hospital with 1600 beds in southern Taiwan from July 2017 to November 2017. Patients with a pyogenic liver abscess as confirmed by abdominal sonography or abdominal CT scans who underwent an ultrasonography- or CT-guided aspiration of an abscess, were enrolled. Clinical data were retrospectively reviewed.
Collection of K. pneumoniae with different serotypes and sites of isolation
During the study period, K. pneumoniae strains from liver abscesses were collected for capsular serotyping by the capsular swelling test, PCR, and ICSs. We also collected both blood broth samples from positive blood culture bottles and the subsequent pure bacterial colony grown on solid media for capsular serotyping with ICSs. Bacterial identification was performed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (bioMérieux, France).
The traditional capsular swelling test for capsular serotypes
Klebsiella pneumoniae strains isolated from pyogenic liver abscesses were subjected to the traditional capsular serotype test. Serotyping was conducted by the capsular swelling technique after inoculation of agar and incubation at 37 °C [2, 4]. Control K. pneumoniae serotypes, including ATCC4208 (K1), were acquired from the American Type Culture Collection (ATCC, Rockville, MD).
PCR for capsular serotyping
Klebsiella pneumoniae isolates from liver abscesses were genotyped for serotypes K1 and K2 by PCR as previously described and were served a reference to compare with the capsular swelling test and ICS results [5, 19, 20]. PCR was carried out to determine the presence of the specific genes for serotypes K1 and K2. The primers specific for K1 consisted of the oligos 5′-GGTGCTCTTTACATCATTGC-3′ and 5′-GCAATGGCCATTTGCGTTAG-3′. K2 primers were 5′-GACCCGATATTCATACTTGACAGAG-3′ and 5′-CCTGAAGTAAAATCGTAAATAGATGGC-3′. Thermocycler conditions were: 1 cycle of denaturation at 94 °C for 1 min, followed by 30 cycles of 94 °C for 30 s, annealing at 58 °C for 45 s and elongation 72 °C for 1 min 30 s and a final extension at 72 °C for 10 min. The amplification was visualised by electrophoresis using 1.5% agarose gel followed by staining in ethidium-bromide.
ICS analysis of the capsular serotype
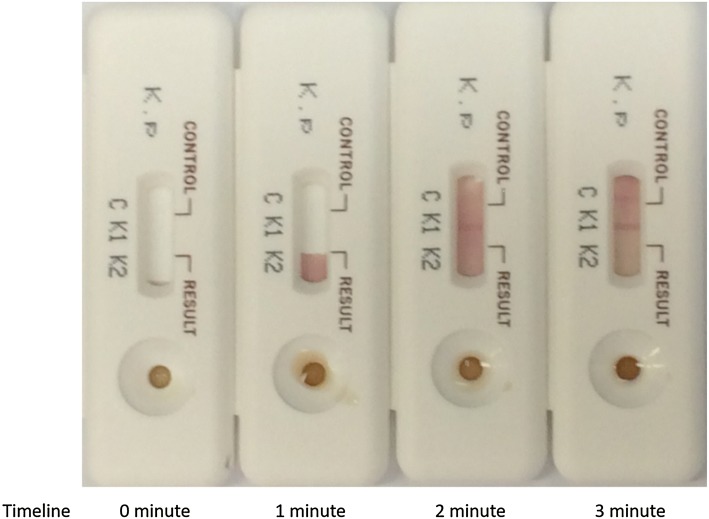
The ICSs were prepared by KeMyth Biotech (Taipei, Taiwan) which had been described before [7]. According to previous study, the ICSs could quickly identify serotypes K1 and K2 K. pneumoniae isolates from single bacterial colony within 5 min. No false-positive or false-negative results were ever seen when testing various serotypes of K. pneumoniae along with other bacterial species. In addition, the ICSs can be stored at room temperature for at least 6 months without losing their sensitivity or specificity [7]. In this study, directly aspirated pus from patients was obtained after an ultrasonography- or CT-guided aspiration procedure. The pus was added into the sample-loading well of the test cassette. If the sample was too mucoid, sterile injection water was mixed with the pus in a 4:1 ratio. Results were read a few minutes later, and this period was chosen as the cut-off point to determine the results. A result obtained after 5 min was considered invalid. In Fig. 3, we demonstrate the procedure and timing for testing by means of ICSs when detecting K. pneumoniae serotype K1.
Fig. 3.
Identification process for serotype K1 K. pneumoniae using ICSs. After the sample was added to the sample loading zone, the red line of serotype K1 (K1) is shown at the second minute. The line of serotype K2 (K2) is not visible in the second minute, and the control line (C) developed in the third minute. Serotype K1 was identified within 3 min. Timeline is indicated at the bottom
PFGE
PFGE typing was performed for 16 K. pneumoniae isolates from pus samples. Total DNA was prepared and digested with XbaI (New England Biolabs Beverly, MA, USA) according to the manufacturer. The restriction fragments were loaded into a 1% agarose gel (Bio-Rad, Hercules, CA, USA) in the running buffer, 0.5 × TBE buffer (45 mM Tris, 45 mM boric acid, and 1.0 mM EDTA [pH 8.0]) with a CHEF Mapper apparatus (Bio-Rad Laboratories, Richmond, CA, USA). The electrophoresis conditions were at 200 V for 24 h, with pulse times of 2–40 s. Gels were stained with ethidium bromide and photographed under UV light.
Capsular serotype detection by ICSs in positive blood culture broth samples and pure bacterial culture
A total of 108 positive flagged blood culture broth samples identified as K. pneumoniae via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and a subsequent pure bacterial colony grown on solid culture media were tested with ICSs for comparison. Besides, another 4 positive flagged blood culture samples caused by bacteria other than K. pneumoniae (2 Gram-positive and 2 Gram-negative microorganisms) were randomly chosen and tested with ICS also. From each blood culture bottle, 120 µl of broth was loaded onto the ICS cassette, and the result was read after 3 min. For pure bacterial culture testing, a single colony of the grown isolate from the blood culture was mixed with 500 µl of sterile saline; 120 µl of the suspension was loaded onto the ICS cassette, and the results were read after 3 min. When inconsistent results were obtained in the comparison of blood culture broth samples with pure bacteria samples by means of ICSs, the wzi gene sequencing method for serotype confirmation was employed [21].
Authors’ contributions
JCL, FYC, and CHW designed the study, collected the clinical data, and wrote the manuscript; PLL and EYML collected and tested the samples; and YYC, FML, and YTL carried out the experiments. All the authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data from the study are available upon request from the corresponding author (JC Lin).
Consent for publication
Not applicable.
Ethics approval and consent to participate
Approval for the study was obtained from the Institutional Review Board at Tri-Service General Hospital (TSGHIRB approval number: 1-106-05-115) and at Kaohsiung Medical University Hospital (KMUHIRB-F(II)20170065 and KMUHIRB-E(II)-20170207).
Funding
This study was supported by grants from the Tri-Service General Hospital and the National Defense Medical Center (TSGH-C105-115, TSGH-C106-095, MAB-105-11, and MAB-106-80).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ICSs
immunochromatographic strips
- PCR
polymerase chain reaction
- PFGE
pulse-field gel electrophoresis
- CT
computed tomography
Contributor Information
Ching-Hsun Wang, Email: sasak0308@gmail.com.
Po-Liang Lu, Email: d830166@gmail.com.
Esther Yip-Mei Liu, Email: eymliu@gmail.com.
Yih-Yuan Chen, Email: yychen@mail.ncyu.edu.tw.
Fu-Mei Lin, Email: zoe@nhri.org.tw.
Yi-Tsung Lin, Email: ytlin8@vghtpe.gov.tw.
Feng-Yee Chang, Email: fychang@mail.ndmctsgh.edu.tw.
Jung-Chung Lin, Email: jclin@ndmctsgh.edu.tw, Email: linjungchung1@yahoo.com.tw.
References
- 1.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 2.Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, Liu CY, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med. 2002;162:1021–1027. doi: 10.1001/archinte.162.9.1021. [DOI] [PubMed] [Google Scholar]
- 4.Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, Wang JJ, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6:1191–1198. doi: 10.1016/j.micinf.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, Ho M, et al. A 5-year study of the seroepidemiology of Klebsiella pneumoniae: high prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J Infect Dis. 2000;181:2075–2079. doi: 10.1086/315488. [DOI] [PubMed] [Google Scholar]
- 6.Onokodi JK, Wauters G. Capsular typing of klebsiellae by coagglutination and latex agglutination. J Clin Microbiol. 1981;13:609–612. doi: 10.1128/jcm.13.4.609-612.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siu LK, Tsai YK, Lin JC, Chen TL, Fung CP, Chang FY. Development of a colloidal gold-based immunochromatographic strip for rapid detection of Klebsiella pneumoniae serotypes K1 and K2. J Clin Microbiol. 2016;54:3018–3021. doi: 10.1128/JCM.01608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbol Rev. 1998;11:589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafat C, Messika J, Barnaud G, Dufour N, Magdoud F, Billard-Pomares T, et al. Hypervirulent Klebsiella pneumoniae, a 5-year study in a French ICU. J Med Microbiol. 2018;67:1083–1089. doi: 10.1099/jmm.0.000788. [DOI] [PubMed] [Google Scholar]
- 10.Lin JC, Chang FY, Fung CP, Yeh KM, Chen CT, Tsai YK, et al. Do neutrophils play a role in establishing liver abscesses and distant metastases caused by Klebsiella pneumoniae? PLoS ONE. 2010;5:e15005. doi: 10.1371/journal.pone.0015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Wong CC, Lai S, Chen H, He X, Sun L, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. 2016;6:38587. doi: 10.1038/srep38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng HP, Chang FY, Fung CP, Siu LK. Klebsiella pneumoniae liver abscess in Taiwan is not caused by a clonal spread strain. J Microbiol Immunol Infect. 2002;2002(35):85–88. [PubMed] [Google Scholar]
- 15.Cheng HP, Siu LK, Chang FY. Extended-spectrum cephalosporin compared to cefazolin for treatment of Klebsiella pneumoniae-caused liver abscess. Antimicrob Agents Chemother. 2003;47:2088–2092. doi: 10.1128/AAC.47.7.2088-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YS, Siu LK, Yeh KM, Fung CP, Huang SJ, Hung HC, et al. Recurrent Klebsiella pneumoniae liver abscess: clinical and microbiological characteristics. J Clin Microbiol. 2009;47:3336–3339. doi: 10.1128/JCM.00918-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, et al. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother. 2014;58:5379–5385. doi: 10.1128/AAC.02523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Ren J, Wang W, Wang G, Gu G, Wu X, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. 2018;37:679–689. doi: 10.1007/s10096-017-3160-z. [DOI] [PubMed] [Google Scholar]
- 19.Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL. Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiella sp. and comparison of isolates within these serotypes. FEMS Microbiol Lett. 2008;284:247–252. doi: 10.1111/j.1574-6968.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 20.Yeh KM, Chang FY, Fung CP, Lin JC, Siu LK. Serotype K1 capsule, rather than magA per se, is really the virulence factor in Klebsiella pneumoniae strains that cause primary pyogenic liver abscess. J Infec Dis. 2006;194:403–404. doi: 10.1086/505153. [DOI] [PubMed] [Google Scholar]
- 21.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decré D. wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51:4073–4078. doi: 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the study are available upon request from the corresponding author (JC Lin).