Abstract
Objective:
Cytokines release by adipocytes could interact with TSH secretion. We evaluated the relationship between adipocytokines and TSH. We further tested for association of cytokines and thyroid autoimmunity.
Methods:
We conducted a cross-sectional study in a community-based sample including 5385 individuals (2964 female) with TSH within the reference range. Subjects who reported taking thyroid medications or drugs that alter thyroid function were excluded. TSH, FT4, adiponectin, leptin, antibody against thyroperoxidase and against thyroglobulin were measured. Linear and logistic regression models were used to test for association.
Results:
Females had higher adiponectin and leptin level and increased frequency of thyroid antibodies. In multiple regression analysis TSH was directly associated with leptin (β = 0.003, p = 0.001) and the presence of circulating antibody against thyroperoxidase (β = 0.315, p < 0.001), but negatively associated with age (β = −0.012, p < 0.001) and FT4 (β = −0.359, p < 0.001). Adiponectin, the presence of antibody against thyroglobulin and smoking habit were not associated with TSH levels (p = 0.223, p = 0.174 and p = 0.788, respectively). Logistic regression analysis revealed that higher adiponectin levels were associated with thyroid autoimmunity.
Conclusions:
Leptin is positively associated with TSH levels in euthyroid individuals, suggesting an effect of the adipokine on TSH secretion. Our results support the hypothesis that the leptin and pituitary-thyroid axis might interact in the context of energy homeostasis. The effect of adiponectin on thyroid autoimmunity will require more studies.
Keywords: TSH within reference range, Thyroid autoimmunity, Adipocytokines, Adiponectin, Leptin
1. Introduction
The past 20 years have witnessed revolutionary change in views of the roles of white adipose tissue (WAT) in the body. Whereas storage and release of lipids remain major functions of adipocytes, adipose tissue is now known to express and secrete a variety of specific lipid molecules for intracellular signalling and to secrete protein molecules (“adipokines”), that act both locally (paracrine, autocrine) and systemically (endocrine) to communicate with essentially every organ system [1]. In addition, adipose tissue is also a major site for the metabolism of sex steroids and glucocorticoids.
It is now well established that excess of adipose tissue, particularly in the viscera, is associated with insulin resistance, diabetes, hypertension, prothrombotic and proinflammatory states, and cardiovascular disease [2,3]. Thus, excess adipose tissue directly contributes to the pathogenesis of obesity-related disorders. Leptin, a 167 amino acid protein, is the best characterized adipocyte-derived hormone [3–5]. This adipokine exerts pleiotropic actions on glucose metabolism, stimulates bone formation [6], regulates immune cell function [7,8], and may promote atherosclerosis and cardiac remodeling [7,9,10]. Adipocytes secrete leptin in proportion to adipose tissue mass as well as nutritional status, and secretion is greater from subcutaneous than visceral adipose tissue [11]. Moreover, this adipokine acts as an afferent satiety signal at a central hypothalamic level.
Leptin also elicits regulatory effects that include interactions with thyroid axis function. In murine fasting models, leptin administration raises thyrotropin (TSH) levels, probably through the stimulation of thyrotropin-releasing hormone (TRH), in the paraventricular nucleus of the hypothalamus [12]. Some studies also suggest that leptin may regulate TSH secretion in humans [13]. Thyroid dysfunction observed in patients with leptin deficiency or leptin receptor abnormality strongly suggests that leptin and the hypothalamic-pituitary-thyroid axis are interacting [14], and leptin may play a role in the peripheral metabolism of thyroid hormones [15]. TSH receptors have also been identified in human adipose tissue, and a direct effect of TSH on leptin secretion by adipose tissue has been reported [3,16,17]. Taken together, the data support the view that leptin may represent a link between thyroid function and adipose tissue mass.
The adipose-specific glycoprotein adiponectin is the most abundant cytokine in adipose tissue and circulates in high concentration, being inversely correlated with visceral obesity and insulin resistance. In contrast to most adipokines, adiponectin is distinguished by its insulin-sensitizing functions. Visceral obesity and type 2 diabetes are associated with low levels of adiponectin [18]. It also displays anti-inflammatory and anti-atherogenic properties [19–22], regulates bone metabolism [23], and protects the heart from ischemia [24].
The relationship between adiponectin and thyroid function, however, is not clearly defined, and only a few studies in humans have been documented. Most studies have been done in hyperthyroid or hypothyroid patients, assuming that the findings were representative of direct effects of thyroid hormones on this adipokine. However, indirect effects have not been excluded, and conclusions have been tentative and even disputed [25,26]. In general, the results do consistently suggest that adiponectin may be upregulated in the hyperthyroid state, but is not modified in hypothyroid subjects.
The aim of our study was to assess whether the relationship between leptin and adiponectin and the thyroid axis in euthyroid individuals. We also tested whether the adipocytokines could be correlated with thyroid autoimmunity
2. Subjects and methods
The cohort is from the SardiNIA study, a population-based survey that investigates several hundreds of phenotypic traits in a longitudinal manner aiming to define the genetic components and the ageing effects involved in their regulation [27–29]. From the initial sample of 6148 individuals, subjects who reported taking thyroid medications (thyroid hormone replacement or thyrostatics) or drugs that alter thyroid function tests (amiodarone, lithium, and corticosteroids) were excluded. For the purpose of the present study, we included only subjects with TSH within the reference range, yielding a final sample of 5385 (aged 14–102 years).
Each participant signed an Informed Consent. All study methods were conducted according to the principles expressed in the Declaration of Helsinki and were approved by the governing Ethics Committee, ASL4.
2.1. Biochemical and hormone assays
Blood venous samples were drawn between 7 and 8 a.m. after an overnight fast and stored at −80 °C until use. Serum samples were assayed for TSH, free thyroxine (FT4), and antibodies against thyroperoxidase (TPOAb) and against thyroglobulin (TGAb) using an automated assay system (Immulite 2000, Siemens, Germany). The method is a twosite, solid-phase chemiluminescent immunometric assay. Normal values were TSH, 0.4–4.0 μIU/ml; FT4, 0.89–1.76 ng/dl; TPOAb, < 35 IU/ml; TGAb, < 40 IU/ml [30].
Leptin and adiponectin (human serum adipokine – panel B; Lincoplex kit: Cat. # HADK2–61 K-B) were measured with a multiplex testing Luminex Model no. Luminex 200 IS Serial No. LX10006265401.
2.2. Statistical analysis
Data are presented as median and interquartile range unless otherwise specified. Multivariable linear regression analysis for continuous variables was conducted to detect associations between TSH and the covariates age, sex, FT4, smoking status, TPOAb and TGAb. Logistic regression models were used to test which of the above variables were predictive of thyroid autoantibodies positivity. A two-sided p value < 0.05 indicated statistical significance in STATA 12.0.
3. Results
Major characteristics of the population sample are described in Table 1. Overall, females were somewhat younger than males and were characterized by lower BMI, higher TSH, and reduced FT4 levels. Females had higher concentration of leptin and adiponectin than males. Table 2 shows the result of multiple regression analysis. TSH had a negative association with age (β = −0.012, p < 0.001) and with FT4 (β = −0.359, p < 0.001). Female sex, the presence of TPOAb, and leptin were positively associated with TSH level (β = 0.150, p < 0.001; β = 0.315, p < 0.001; β = 0.003, p = 0.001, respectively). By contrast, BMI, the presence of TGAb, and smoking habits were not associated with TSH (p = 0.146, p = 0.174 and p = 0.788, respectively).
Table 1.
Summary characteristics of the sample.
| Female (n = 2964) | Male (n = 2421) | Total (n = 5385) | |
|---|---|---|---|
| Age, yrs. | 40.4 (28.6–55.8) | 42.2 (29.4–57.8) | 41.4 (28.8–56.5)* |
| BMI. kg/m2 | 23.6 (20.8–27.1) | 25.9 (23.3–28.6) | 24.7 (21.8–28.0)** |
| TSH, mUI/ml | 1.69 (1.13–2.34) | 1.46 (1.01–2.03) | 1.59 (1.06–2.20)** |
| FT4, ng/dl | 1.28 (1.17–1.40) | 1.29 (1.18–1.42) | 1.29 (1.18–1.41)* |
| Adiponectin, mg/dl | 2.78 (1.94–3.91) | 1.81 (1.27–2.68) | 2.33 (1.52–3.42)** |
| Leptin, ng/ml | 7.86 (3.61–13.70) | 3.22 (1.33–6.07) | 5.14 (2.11–10.60)** |
| TPOAb, n (%) | 393 (13.3%) | 155 (6.4%) | 548 (10.2%)** |
| TGAb, n (%) | 390 (13.2%) | 132 (5.5%) | 522 (9.7%)** |
| TGAb or TPOAb, n (%) | 593 (20.0%) | 227 (9.4%) | 820 (15.2%)** |
| Smokers, n (%) | 446 (15.1%) | 666 (27.5%) | 1112 (20.7%)** |
Data are expressed as median (25–75°); n, absolute number.
Abbreviations: BMI, body mass index; FT4, free thyroxine; TSH, thyrotropin; TPOAb, antibodies against thyroperoxidase; TGAb, antibodies against thyroglobulin.
Female vs. male, p < 0.05.
Female vs. male, p < 0.001.
Table 2.
Result of multiple regression analysis. Variables associated with TSH.
| β | SE | t | p value | |
|---|---|---|---|---|
| Age | −0.012 | 0.001 | −16.7 | < 0.001 |
| Female | 0.150 | 0.024 | −6.2 | < 0.001 |
| BMI | −0.004 | 0.003 | −1.5 | 0.146 |
| FT4 | −0.359 | 0.058 | −6.3 | < 0.001 |
| Adiponectin | −0.007 | 0.006 | −1.2 | 0.223 |
| Leptin | 0.003 | 0.001 | 2.9 | 0.004 |
| TPOAb | 0.315 | 0.038 | 8.2 | < 0.001 |
| TGAb | 0.053 | 0.039 | 1.4 | 0.174 |
| Smoke | 0.007 | 0.027 | 0.3 | 0.788 |
Abbreviations: β, beta coefficient; SE, standard error; t, t score; BMI, body mass index; TPOAb, antibodies against thyroperoxidase; TGAb, antibodies against thyroglobulin.
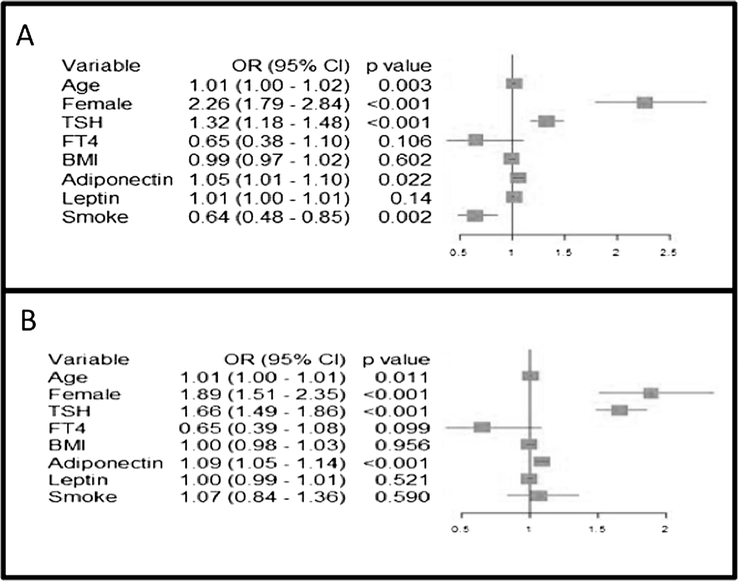
Logistic regression analysis for the presence of TPOAb showed that age, female sex, TSH, and adiponectin were predictors of antibody positivity (respectively: OR 1.01, 95% CI = 1.00–1.01, p = 0.011, OR 1.89, 95% CI = 1.51–2.35, p < 0.001; OR 1.66, 95% CI = 1.49–1.86, p < 0.001; OR 1.09, 95% CI = 1.05–1.14, p < 0.001). BMI, FT4, smoke, and leptin were not associated with the presence of circulating TPOAb (p = 0.099 or higher) (Fig. 1, panel A).
Fig. 1.
Panel A: Variables associated with the presence of antibodies against thyroperoxidase: result of logistic regression analysis. Panel B: Variables associated with the presence of antibodies against thyroglobulin: result of logistic regression analysis.
The variables associated with TGAb positivity were again age, female sex, TSH, adiponectin, and smoking (respectively: OR 1.01, 95% CI = 1.00–1.01, p = 0.003, OR 2.26, 95% CI = 1.79–2.84, p < 0.001; OR 1.32, 95% CI = 1.18–1.48, p < 0.001; OR 1.05, 95% CI = 1.01–1.10, p = 0.022; OR 0.64, 95% CI = 0.48–0.85, p = 0.002), as shown in Fig. 1 panel B. BMI, FT4, and leptin showed no effect.
4. Discussion
Thyroid hormone exerts profound effects on lipid metabolism [31–33], cardiovascular system [34,35], and could have a role in metabolic syndrome [36]. Because leptin and thyroid hormones have similar effect on “body homeostasis”, several earlier studies have explored possible relations between their circulating levels in humans and experimental animals, but somewhat discordant results. Serum leptin concentration was reported to be elevated in women with subclinical or overt hypothyroidism, and levothyroxine treatment reduced leptin levels in these patients [37]. An additional study found leptin levels lower in hyperthyroidism, and thyrostatic treatment increased leptin level [38]. However, another study failed to confirm these findings [39]. The variable results may be related to the multiple sites of action of leptin, with a complex interaction with the pituitary-thyroid axis. Leptin modulates TRH gene expression in the paraventricular nucleus if the hypothalamus [40], and specific receptors have been demonstrated in the pituitary [41,42]. In addition, the subcutaneous administration of leptin significantly blunted the fall of TSH and partially reversed the fall in thyroid hormones induced by prolonged fasting [43].
In this cross-sectional study we tried to avoid some confounders, including some genetic variability, by focusing on euthyroid individuals in members of the founder population of Sardinia. We found that leptin level was associated with TSH level in 5385 euthyroid individuals. The effects, however, are likely to be bidirectional. Indeed, functioning TSH receptors have been demonstrated on the surface of white adipocytes [44] and changes in plasma TSH have been reported to contribute to the regulation of leptin pulses [45]. Additionally, it has been demonstrated that the administration of recombinant TSH can induce a significant leptin release which is proportional to the adipose tissue mass [46]. Thus, our findings suggest that a fall in circulating leptin levels might likely act, at the hypothalamic level, as an inhibitory signal to reduce the activity of the hypothalamic-pituitary-thyroid axis. Speculatively, this feedback loop may have originated to help conserve energy in conditions of food shortage or in response to severe illness associated with a low triiodothyronine syndrome.
When we extended the study to antithyroid autoantibodies, we further found that higher leptin levels also correlated with more prevalent thyroid autoimmunity in women [47,48]. Moreover, the median leptin in the entire sample was significantly higher in subjects with circulating TPOAb and TGAb compared to antibody-negative subjects. However, logistic regression analyses for the presence of antibodies against thyroid showed age, female sex and TSH as predictors but neither leptin nor BMI or FT4 were associated with the presence of any circulating antibody. Nevertheless, a previous study reported that leptin was capable of modulating the immune response, and hyperleptinemia has been associated with an increased susceptibility to autoimmune diseases by stimulating proinflammatory cytokines and macrophages [49]. Overall, a clear clinical association between hyperleptinemia and autoimmune disease is not established to date.
We also found a positive and significant correlation between levels of adiponectin and markers of thyroid autoimmunity (TPOAb and TGAb) in these euthyroid individuals, consistent with suggestions of an important role of adiponectin in innate and autoimmune phenomena [50] and a possible role in autoimmune thyroid disease. Apart from its pleiotropic metabolic functions, whether adiponectin is a protective or a detrimental cytokine in autoimmune disease is still under discussion. Anti-inflammatory activities of adiponectin range from reports of induction of anti-inflammatory cytokines to inhibition of pro-inflammatory cytokines status [24,51]. A growing body of evidence points to upregulation of this cytokine in several inflammatory and immune-mediated conditions. For instance, elevated circulating levels of adiponectin have been reported in patients with autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematous and autoimmune diabetes mellitus [51,52]. In some human studies an adiponectin thyroid hormone interaction have been inferred [53]. In particular, elevation of adiponectin levels and positive correlation with FT4 and free triiodothyronine or TSH receptor antibodies was found in hyperthyroid Graves’ disease patients [54]. On the other hand, multiple lines of evidence indicate that inflammation inhibits adiponectin production through several mechanisms [55]. But it may be that other - factors overcome a suppressive effect of inflammation on adiponectin production, For example, because adiponectin forms oligomers (trimer, hexamer and high molecular weight forms) and circulates in plasma as truncated fragments [56], different results obtained in various experiments may speculatively be related to the various forms of adiponectin used. The exact mechanisms leading to the significant association we observed, and the specific role of adiponectin in the pathophysiology of TPOAb and TGAb production, however, are thus conjectural and require further mechanistic studies.
The strengths of our study include a well-characterized, large community-based sample, which has sufficient statistical power to draw consistent correlations between variables. We also excluded participants with serum TSH levels outside the range considered physiological. However, we acknowledge limitations: subjects had only one assessment of TSH, thus potentially including individuals with nonthyroidal illness or transient abnormalities. Further, thyroid hormone profile is not completed, as we did not assess serum FT3 level, and data on ultrasonography of thyroid gland is lacking. Finally, the cross sectional design of the study precludes causal inferences.
In conclusion, the present findings indicate that leptin is positively associated with TSH levels in euthyroid individuals. TSH plasma values were higher in individuals with higher leptin levels suggests a direct effect of the adipokine. Thus, our results support the hypothesis that leptin and the pituitary-thyroid axis might interact for energy homeostasis. We also reported an association between adiponectin and autoantibodies against thyroid antigen which suggest a role of adiponectin in autoimmune thyroiditis. This hypothesis should be tested by future specific studies.
Acknowledgments
6. Funding
This work was supported by the National Institute on Aging [Contract NO1-AG-1–2109] and in part by the Intramural Research Program of the NIH, National Institute on Aging, US, Contract NO1-AG-1–2109.
Footnotes
5. Declaration of interest
This research did not receive any specific grant from funding agencies in the public, commercial, ornot-for-profit sectors
References
- [1].Lafontan M, Adipose tissue and adipocyte dysregulation, Diab. Metabol 40 (2014) 16–28. [DOI] [PubMed] [Google Scholar]
- [2].Guerra S, Boscari F, Avogaro A, Di Camillo B, Sparacino G, de Kreutzenberg SV, Hemodynamics assessed via approximate entropy analysis of impedance cardio-graphy time series: effect of metabolic syndrome, Am. J. Physiol. Heart Circul. Physiol 301 (2011) H592–H598. [DOI] [PubMed] [Google Scholar]
- [3].Herder C, Kannenberg JM, Niersmann C, Huth C, Carstensen-Kirberg M, Wittenbecher C, et al. , Independent and opposite associations of serum levels of omentin-1 and adiponectin with increases of glycaemia and incident type 2 diabetes in an older population: KORA F4/FF4 study, Eur. J. Endocrinol 177 (2017) 277–286. [DOI] [PubMed] [Google Scholar]
- [4].Friedman JM, Halaas JL, Leptin and the regulation of body weight in mammals, Nature 395 (1998) 763–770. [DOI] [PubMed] [Google Scholar]
- [5].Bluher M, Mantzoros CS, From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century, Metabol. Clin. Exper 64 (2015) 131–145. [DOI] [PubMed] [Google Scholar]
- [6].Upadhyay J, Farr OM, Mantzoros CS, The role of leptin in regulating bone metabolism, Metabol. Clin. Exper 64 (2015) 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Procaccini C, Pucino V, Mantzoros CS, Matarese G, Leptin in autoimmune diseases, Metabol. Clin. Exper 64 (2015) 92–104. [DOI] [PubMed] [Google Scholar]
- [8].La A, Cava Leptin in inflammation and autoimmunity, Cytokine 98 (2017) 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scuteri A, Orru M, Morrell C, Piras MG, Taub D, Schlessinger D, et al. , Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study, Atherosclerosis 215 (2011) 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farcas AD, Rusu A, Stoia MA, Vida-Simiti LA, Plasma leptin, but not resistin, TNF-alpha and adiponectin, is associated with echocardiographic parameters of cardiac remodeling in patients with coronary artery disease, Cytokine 103 (2018) 46–49. [DOI] [PubMed] [Google Scholar]
- [11].Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW, Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans, Endocrinology 145 (2004) 2273–2282. [DOI] [PubMed] [Google Scholar]
- [12].Duntas LH, Biondi B, The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin, Thyroid: official journal of the American Thyroid Association 23 (2013) 646–653. [DOI] [PubMed] [Google Scholar]
- [13].Feldt-Rasmussen U, Thyroid and leptin, Thyroid : official journal of the American Thyroid Association 17 (2007) 413–419. [DOI] [PubMed] [Google Scholar]
- [14].Korbonits M, Leptin and the thyroid–a puzzle with missing pieces, Clin. Endocrinol 49 (1998) 569–572. [DOI] [PubMed] [Google Scholar]
- [15].Reinehr T, Obesity and thyroid function, Molecul. Cell. Endocrinol 316 (2010) 165–171. [DOI] [PubMed] [Google Scholar]
- [16].Sorisky A, Antunes TT, Gagnon A, The Adipocyte as a novel TSH target, Mini Rev. Med. Chem 8 (2008) 91–96. [DOI] [PubMed] [Google Scholar]
- [17].Heinen CA, Zhang Z, Klieverik LP, de Wit TC, Poel E, Yaqub M, et al. , Effects of intravenous thyrotropin-releasing hormone on (18)F-fluorodeoxyglucose uptake in human brown adipose tissue: a randomized controlled trial, Eur. J. Endocrinol 179 (2018) 31–38. [DOI] [PubMed] [Google Scholar]
- [18].Li S, Shin HJ, Ding EL, van Dam RM, Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis, Jama 302 (2009) 179–188. [DOI] [PubMed] [Google Scholar]
- [19].Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, et al. , Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: a systematic review and meta-analyses, Metabol. Clin. Exp 69 (2017) 51–66. [DOI] [PubMed] [Google Scholar]
- [20].Gasbarrino K, Gorgui J, Nauche B, Cote R, Daskalopoulou SS, Circulating adiponectin and carotid intima-media thickness: a systematic review and meta-analysis, Metabol. Clin. Exper 65 (2016) 968–986. [DOI] [PubMed] [Google Scholar]
- [21].Tesauro M, Canale MP, Rodia G, Di Daniele N, Lauro D, Scuteri A, et al. , Metabolic syndrome, chronic kidney, and cardiovascular diseases: role of adipokines, Cardiol. Res. Pract 2011 (2011) 653182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Menezes AMB, Oliveira PD, Wehrmeister FC, Goncalves H, Assuncao MCF, Tovo-Rodrigues L, et al. , Association between interleukin-6, C-reactive protein and adiponectin with adiposity: findings from the 1993 pelotas (Brazil) birth cohort at 18 and 22years, Cytokine 110 (2018) (1993) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pal China S, Sanyal S, Chattopadhyay N, Adiponectin signaling and its role in bone metabolism, Cytokine (2018). [DOI] [PubMed] [Google Scholar]
- [24].Villarreal-Molina MT, Antuna-Puente B, Adiponectin: anti-inflammatory and cardioprotective effects, Biochimie 94 (2012) 2143–2149. [DOI] [PubMed] [Google Scholar]
- [25].Iglesias P, Alvarez Fidalgo P, Codoceo R, Diez JJ, Serum concentrations of adipocytokines in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function, Clin. Endocrinol 59 (2003) 621–629. [DOI] [PubMed] [Google Scholar]
- [26].Santini F, Marsili A, Mammoli C, Valeriano R, Scartabelli G, Pelosini C, et al. , Serum concentrations of adiponectin and leptin in patients with thyroid dysfunctions, J. Endocrinol. Invest 27 (2004) RC5–7. [DOI] [PubMed] [Google Scholar]
- [27].Scuteri A, Morrell CH, Orru M, Strait JB, Tarasov KV, Ferreli LA, et al. , Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging, Hypertension 64 (2014) 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zoledziewska M, Sidore C, Chiang CWK, Sanna S, Mulas A, Steri M, et al. , Height-reducing variants and selection for short stature in Sardinia, Nat. Genet 47 (2015) 1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pani A, Bragg-Gresham J, Masala M, Piras D, Atzeni A, Pilia MG, et al. , Prevalence of CKD and its relationship to eGFR-related genetic loci and clinical risk factors in the SardiNIA study cohort, J. Am. Soc. Nephrol.: JASN 25 (2014) 1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Delitala AP, Filigheddu F, Orru M, AlGhatrif M, Steri M, Pilia MG, et al. , No evidence of association between subclinical thyroid disorders and common carotid intima medial thickness or atherosclerotic plaque, Nutr. Metabol. Cardiovasc. Diseases: NMCD 25 (2015) 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Delitala AP, Delitala G, Sioni P, Fanciulli G, Thyroid hormone analogs for the treatment of dyslipidemia: past, present, and future, Curr. Med. Res. Opin 33 (2017) 1985–1993. [DOI] [PubMed] [Google Scholar]
- [32].Delitala AP, Fanciulli G, Maioli M, Delitala G, Subclinical hypothyroidism, lipid metabolism and cardiovascular disease, Eur. J. Inter. Med 38 (2017) 17–24. [DOI] [PubMed] [Google Scholar]
- [33].Delitala AP, Steri M, Pilia MG, Dei M, Lai S, Delitala G, et al. , Menopause modulates the association between thyrotropin levels and lipid parameters: the SardiNIA study, Maturitas 92 (2016) 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Delitala AP, Subclinical hyperthyroidism and the cardiovascular disease, Hormone Metabol. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 49 (2017) 723–731. [DOI] [PubMed] [Google Scholar]
- [35].Delitala AP, Orru M, Filigheddu F, Pilia MG, Delitala G, Ganau A, et al. , Serum free thyroxine levels are positively associated with arterial stiffness in the SardiNIA study, Clin. Endocrinol 82 (2015) 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Delitala AP, Fanciulli G, Pes GM, Maioli M, Delitala G, Thyroid hormones, metabolic syndrome and its components, Endocrine Metabol. Immune Disorders Drug Targets 17 (2017) 56–62. [DOI] [PubMed] [Google Scholar]
- [37].Teixeira PF, Cabral MD, Silva NA, Soares DV, Braulio VB, Couto AP, et al. , Serum leptin in overt and subclinical hypothyroidism: effect of levothyroxine treatment and relationship to menopausal status and body composition, Thyroid: official journal of the American Thyroid Association 19 (2009) 443–450. [DOI] [PubMed] [Google Scholar]
- [38].Oge A, Bayraktar F, Saygili F, Guney E, Demir S, TSH influences serum leptin levels independent of thyroid hormones in hypothyroid and hyperthyroid patients, Endocrine J 52 (2005) 213–217. [DOI] [PubMed] [Google Scholar]
- [39].Matsubara M, Yoshizawa T, Morioka T, Katayose S, Serum leptin and lipids in patients with thyroid dysfunction, J. Atherosclerosis Thrombos 7 (2000) 50–54. [DOI] [PubMed] [Google Scholar]
- [40].Guo F, Bakal K, Minokoshi Y, Hollenberg AN, Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo, Endocrinology 145 (2004) 2221–2227. [DOI] [PubMed] [Google Scholar]
- [41].Lloyd RV, Jin L, Tsumanuma I, Vidal S, Kovacs K, Horvath E, et al. , Leptin and leptin receptor in anterior pituitary function, Pituitary 4 (2001) 33–47. [DOI] [PubMed] [Google Scholar]
- [42].Cabanelas A, Lisboa PC, Moura EG, Pazos-Moura CC, Leptin acute modulation of the 5’-deiodinase activities in hypothalamus, pituitary and brown adipose tissue of fed rats, Hormone Metabol. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 38 (2006) 481–485. [DOI] [PubMed] [Google Scholar]
- [43].Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. , Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight, J. Clin. Invest 115 (2005) 3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sorisky A, Bell A, Gagnon A, TSH receptor in adipose cells, Hormone Metabol. Res. = Hormon- und Stoffwechselforschung = Hormones et metabolisme 32 (2000) 468–474. [DOI] [PubMed] [Google Scholar]
- [45].Flier JS, Harris M, Hollenberg AN, Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring, J. Clin. Invest 105 (2000) 859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Santini F, Galli G, Maffei M, Fierabracci P, Pelosini C, Marsili A, et al. , Acute exogenous TSH administration stimulates leptin secretion in vivo, Eur. J. Endocrinol 163 (2010) 63–67. [DOI] [PubMed] [Google Scholar]
- [47].Delitala AP, Pilia MG, Ferreli L, Loi F, Curreli N, Balaci L, et al. , Prevalence of unknown thyroid disorders in a Sardinian cohort, Eur. J. Endocrinol 171 (2014) 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Delitala AP, Terracciano A, Fiorillo E, Orru V, Schlessinger D, Cucca F, Depressive symptoms, thyroid hormone and autoimmunity in a population-based cohort from Sardinia, J. Affective Disorders 191 (2016) 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fresno M, Alvarez R, Cuesta N, Toll-like receptors, inflammation, metabolism and obesity, Arch. Physiol. Biochem 117 (2011) 151–164. [DOI] [PubMed] [Google Scholar]
- [50].Peake PW, Shen Y, Walther A, Charlesworth JA, Adiponectin binds C1q and activates the classical pathway of complement, Biochem. Biophys. Res. Commun 367 (2008) 560–565. [DOI] [PubMed] [Google Scholar]
- [51].Toussirot E, Binda D, Gueugnon C, Dumoulin G, Adiponectin in autoimmune diseases, Curr. Med. Chem 19 (2012) 5474–5480. [DOI] [PubMed] [Google Scholar]
- [52].Delitala AP, Sanciu FA, Errigo A, Delitala G, Pes GM, Leptin levels and insulin dependence in latent autoimmune diabetes in adults, J. Interfer. Cytokine Res.: the official journal of the International Society for Interferon and Cytokine Research 37 (2017) 550–556. [DOI] [PubMed] [Google Scholar]
- [53].Hsieh CJ, Wang PW, Serum concentrations of adiponectin in patients with hyperthyroidism before and after control of thyroid function, Endocrine J. 55 (2008) 489–494. [DOI] [PubMed] [Google Scholar]
- [54].Saito T, Kawano T, Saito T, Ikoma A, Namai K, Tamemoto H, et al. , Elevation of serum adiponectin levels in Basedow disease, Metabol. Clin. Exper 54 (2005) 1461–1466. [DOI] [PubMed] [Google Scholar]
- [55].Wood IS, Stezhka T, Trayhurn P, Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension, Pflugers Archiv : Eur. J. Physiol 462 (2011) 469–477. [DOI] [PubMed] [Google Scholar]
- [56].Liu M, Liu F, Regulation of adiponectin multimerization, signaling and function, Best Practice Res.Clin. Endocrinol. Metabol 28 (2014) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]