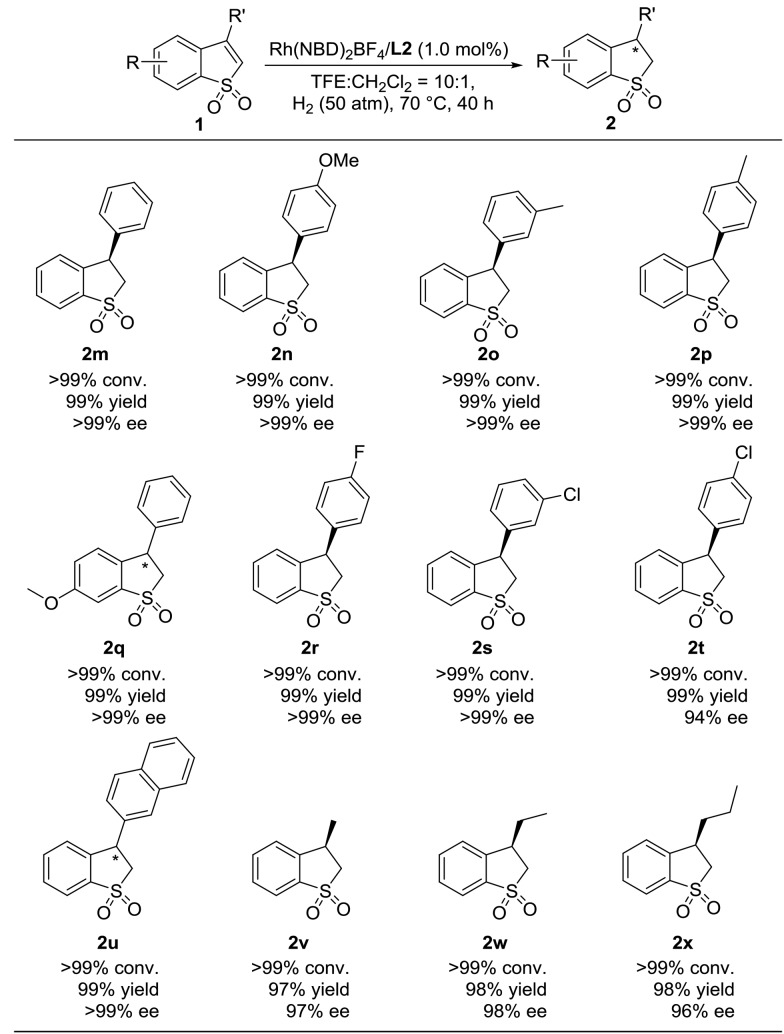
Table 4. Scope study of the Rh-catalyzed asymmetric hydrogenation of 3-substituted benzo[b]thiophene 1,1-dioxides a .
|
a0.1 mmol substrate 1, substrate 1/Rh(NBD)2BF4/L2 = 1/0.01/0.011 at 70 °C under 50 atm H2 in 1.0 mL CF3CH2OH for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial). Conversion was determined by 1H NMR analysis. Yield is isolated yield. The ee value was determined by HPLC on a chiral column. The absolute configurations of 2n and 2v were determined as (R) according to previous work.7c
