Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- APRI
aspartate aminotransferase‐to‐platelet ratio index
- AST
aspartate aminotransferase
- CT
computed tomography
- FALD
Fontan‐associated liver disease
- GGT
gamma‐glutamyltranspeptidase
- HCC
hepatocellular carcinoma
- HT
heart transplant
- INR
international normalized ratio
- LC
liver cirrhosis
- LF
liver fibrosis
- LHT
liver‐heart transplant
- LS
liver stiffness
- MELD
Model of End‐stage Liver Disease
- MRE
magnetic resonance elastography
- MRI
magnetic resonance imaging
- PELD
Pediatric End‐Stage Liver Disease
- PT
prothrombin time
Since its introduction more than 40 years ago, the Fontan procedure, which redirects venous blood flow directly to pulmonary circulation to palliate single‐ventricle physiology, has enabled many children to survive to adulthood. Their improved prognosis has unveiled a multitude of complications related to the unique Fontan physiology. Fontan‐associated liver disease (FALD) is a process universal to the post‐Fontan population and includes a spectrum of pathology that ranges from mild liver fibrosis (LF) to liver cirrhosis (LC) and hepatocellular carcinoma (HCC).1, 2 We will review advances in the field and their implications for patient management.
Areas of Focus in Fontan‐Associated Liver Disease Clinical Practice and Research
Clinical manifestations of liver disease in the post‐Fontan population are variable. Some patients with FALD may be entirely asymptomatic, or have only mild nonspecific symptoms of compensated cirrhosis (Table 1). Others may present with common signs and symptoms of severe decompensated disease. Several clinical, laboratory, and imaging modalities to assess LC are summarized in Table 1; however, liver biopsy remains the gold standard for diagnosis.3
Table 1.
Modalities to Diagnose Liver Cirrhosis
| Category | Modality | Findings |
|---|---|---|
| Physician preliminary evaluation | Symptoms assessment |
• Anorexia • Fatigue • Weight loss |
| Physical examination |
• Jaundice • Palmar erythema • Splenomegaly • Asterixis • Ascites (may appear secondary to heart failure without cirrhosis) • Spider nevi • Clubbing • Gynecomastia • Caput medusa |
|
| Laboratory evaluation | (1) APRI scorea |
APRI < 1.0: cirrhosis is unlikely APRI > 2.0: suggests presence of cirrhosis |
| (2) FIB‐4 scoreb |
FIB‐4 < 1.45: cirrhosis is unlikely FIB‐4 < 3.25: suggests presence of cirrhosis |
|
| Radiology | Ultrasonography |
• Nodular liver surface • Irregular borders • Increased echogenicity • Splenomegaly |
| CT | Reticular hepatic enhancement during portal venous phase | |
| MRE | Score >4.9 diagnostic for cirrhosis in other populations; not validated in FALD because of confounding hepatic congestion increasing stiffness scores | |
| Shear wave elastography | Measures liver stiffness as surrogate for fibrosis | |
| Pathology | Liver biopsy | Gold standard for diagnosis; generally not required if other tests described earlier are confirmatory of cirrhosis |
Aspartate aminotransferase (AST)‐to‐platelet ratio index (APRI) score = (AST [IU/L]/AST upper limit of normal [IU/L] × 100)/platelet count (×109/L).
Fibrosis‐4 (FIB‐4) score = age (years) × (AST [IU/L]/AST upper limit of normal [IU/L] × alanine aminotransferase [IU/L])1/2/platelet count (×109/L).
Adapted with permission from Congenital Heart Disease.3 Copyright 2017, Wiley Periodicals, Inc.
The two main areas of focus in clinical practice and research are the standardization of histological grading of fibrosis and the identification of noninvasive modalities to assess LF and LC.1, 4, 5 Their common goal is to help risk‐stratify this population and identify opportunity for early intervention in those at risk for the severe sequelae, LC, and HCC.6
Novel Histological Grading
Recent publications have attempted to tailor a histological grading system, evaluating the unique disease progression of both portal and sinusoidal fibrosis in FALD. Hypoxia both pre‐ and post‐Fontan secondary to low cardiac output, continuous nonpulsatile hepatic congestion, and increased mesenteric vascular resistance all contribute to the FALD pattern of fibrosis (Fig. 1).3 A fibrosis score using multiple grading systems and a quantitative percent collagen deposition score have been suggested to create a common language between the pathologist and hepatologist.1, 5 Use of sirius red staining, with digital analysis to calculate a quantitative fibrosis score, described by Surrey et al.,5 is an example of a novel approach to histological analysis in FALD (Table 2).4 This may facilitate timely identification of changes in liver architecture, which could have implications for treatment.
Figure 1.
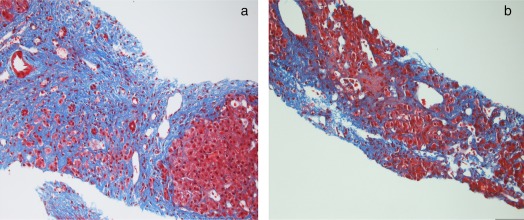
FALD histology. Trichome stains from a patient 14 years post‐Fontan demonstrating (A) portal area replaced by expanded band of fibrosis resulting in nodule and (B) fibrosis extending from portal tracts with pericellular fibrosis in the areas of dilated sinusoids. (Images courtesy of Michelle R. Ewart, M.D.)
Table 2.
Suggested Histological Grading System for Fontan‐Associated Liver Disease
| Scoring System | Range of Scoring | Use |
|---|---|---|
| Metavir | F0‐F4 | Is used to evaluate fibrosis originating in the portal system, which differentiates FALD fibrosis from congestive hepatopathy |
| Congestive hepatic fibrosis score | 0‐4 | A scoring system commonly used for congestive hepatopathy; used to evaluate congestive component of FALD |
| Sinusoidal dilation score | 0‐3 | Scores the percent of sinusoidal dilation present on histology |
| Sinusoidal fibrosis score | 0‐3 | Scores the percent of sinusoidal fibrosis present on histology |
Describes the use of multiple conjoined fibrosis scores to address the unique natural history of fibrosis.
Adapted from Human Pathology.5 Copyright 2016, Elsevier.
Serum Markers
Several studies over the past decade tried to correlate certain serum markers with disease progression, but they had inconsistent results and small cohorts.7 Aminotransferases may be normal or only mildly elevated in FALD. Gamma‐glutamyltranspeptidase (GGT) was mildly elevated (median 69 U/L) in 75% of patients in a study by Wu et al.,1 but was not correlated to histological progression of disease. Elevated prothrombin time (PT/international normalized ratio [INR]) may be a marker for disease progression, as shown by Surrey et al.5 to be correlated with high‐grade fibrosis scores. A recent series evaluating a validated tool for fibrosis in adult populations from other etiologies, FibroSure (LabCorp, Burlington, NC), did not show significant correlation to progression of disease.8 There are no official guidelines, and evaluation is institution dependent (Fig. 2).
Figure 2.
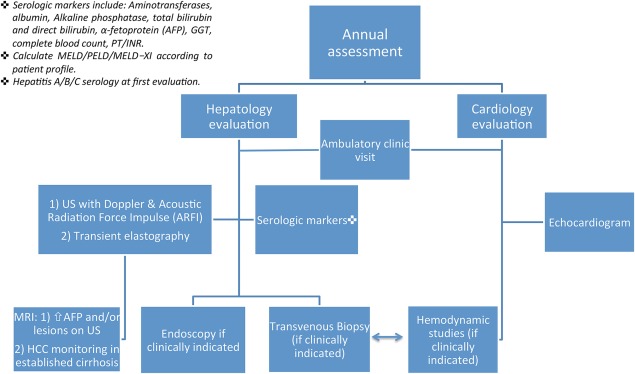
Authors' institutional algorithm for FALD patient evaluation. Abbreviations: MRI, magnetic resonance imaging; PELD, Pediatric End‐Stage Liver Disease.
Radiological Monitoring
Most patients with FALD will have some abnormal finding on routine imaging. Heterogeneous enhancement of liver parenchyma and irregular liver contour are seen in up to 98% and 50% of patients, respectively, across all imaging including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).1, 7, 8, 9, 10 Correlations between radiological findings and progression of LF on histology have inconsistent results among various studies.1, 5, 7 The use of diffusion‐weighted imaging has recently been described as a research tool to evaluate liver microperfusion in FALD. Results have shown its utility in differentiating the unique histopathological changes in the portal circulation of FALD causing cirrhosis (Fig. 1) from those caused by congestive hepatopathy from other cardiac etiologies.11 However, this modality has yet to be clinically validated.
There is inconsistency between institutional practices of radiological monitoring.1, 6, 7, 10, 12 Annual ultrasound imaging appears to be the consensus in the studies detailed earlier and is also the common practice in our institution. MRI is the preferred modality to monitor patients with confirmed cirrhosis who are at risk for development of HCC.6, 7
Elastography
Elastography is a tool validated in the adult population for monitoring chronic LF in various noncardiac causative factors.7, 10, 12 Liver stiffness (LS) scores are described in kilopascals. Hepatic congestion may increase LS scores unrelated to progression of fibrosis. This may be a confounding variable while trying to standardize LS scores, which are based on other etiologies of fibrosis such as hepatitis. However, LS scores are elevated at baseline in FALD in both transient elastography and magnetic resonance elastography (MRE), even compared with other causes of congestive hepatopathy. MRE has shown promising results from a single‐center study as a screening tool for HCC.12 The main limitation of studies to date is lack of data correlating LS scores to histology and cardiac hemodynamics prospectively over time. Our group is trying to bridge this knowledge gap, as are other institutions around the world, to help assess whether these exciting new modalities may be useful as monitoring tools.
Implications for Treatment
The main hepatology consideration when assessing a patient for either heart transplant (HT) or combined liver‐heart transplant (LHT) is the ability of the liver to sustain such complex procedures. In a review by Greenway et al.,6 the authors recommended that patients suffering from failing Fontan physiology, with low Child‐Pugh class A score (<7) and Model of End‐stage Liver Disease (MELD) score (<12), be listed for HT (1‐year posttransplant survival rate of 80%). Pharmacological treatments being considered are phospodiesterase‐5 inhibitors, such as sildenafil, which improve pulmonary vasodilation and reduce afterload in hepatic vein, or renin‐angiotensin system inhibitors, which have demonstrated antifibrotic properties in addition to their reduction of systemic afterload.5 The hepatologist's role is to recognize liver findings suggestive of compromised Fontan circulation and decrease in cardiac output in patients who may benefit from interventional or surgical revision of Fontan, possible HT, or combined LHT (Fig. 3).
Figure 3.
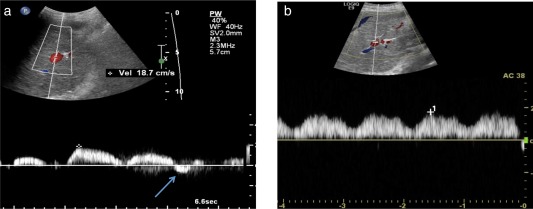
Restoration of portal vein flow after cardiac catheterization intervention. (A) Portal flow reversal seen (arrow) in a 17‐year‐old male with severe portal hypertension and esophageal variceal bleeding found to have a compromised Fontan circulation secondary to constriction of baffle. (B) Normalization of flow in portal vein on Doppler, 2 weeks after stenting of baffle restoring Fontan circulation.
Summary
FALD continues to attract much research interest as complications affecting patient morbidity and mortality become increasingly apparent. Currently, there are still no official guidelines for monitoring this population, and multicenter cohort studies have only been published within the past year.1 Guidelines are institution dependent (Fig. 2, Table 1).1, 3, 7, 10, 12 Future research will continue to focus on risk stratification of patients for severe sequelae of liver disease and on understanding the role of elastography and noninvasive markers in this population while trying to learn more about the pathophysiological etiology of FALD.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, et al. Liver health in adults with Fontan circulation: a multicenter cross‐sectional study. J Thorac Cardiovasc Surg 2017;153:656‐664. [DOI] [PubMed] [Google Scholar]
- 2. Romero R. Liver in congenital heart disease: implications of the fontan procedure for pediatric and adult liver specialists. Clin Liver Dis 2013;2:210‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hilscher MB, Johnson JN, Cetta F, Driscoll DJ, Poterucha JJ, Sanchez W, et al. Surveillance for liver complications after the Fontan procedure. Congenit Heart Dis 2017;12:124‐132. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, et al. Hepatic fibrosis is universal following fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc 2017;6:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Surrey LF, Russo P, Rychik J, Goldberg DJ, Dodds K, O'Byrne ML, et al. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol 2016;57:106‐115. [DOI] [PubMed] [Google Scholar]
- 6. Greenway SC, Crossland DS, Hudson M, Martin SR, Myers RP, Prieur T, et al. Fontan‐associated liver disease: Implications for heart transplantation. J Heart Lung Transplant 2016;35:26‐33. [DOI] [PubMed] [Google Scholar]
- 7. Bradley E, Hendrickson B, Daniels C. Fontan liver disease: review of an emerging epidemic and management options. Curr Treat Options Cardiovasc Med 2015;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fidai A, Dallaire F, Alvarez N, Balon Y, Clegg R, Connelly M, et al. Non‐invasive investigations for the diagnosis of Fontan‐associated liver disease in pediatric and adult fontan patients. Front Cardiovasc Med 2017;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu FM, Jonas MM, Opotowsky AR, Harmon A, Raza R, Ukomadu C, et al. Portal and centrilobular hepatic fibrosis in Fontan circulation and clinical outcomes. J Heart Lung Transplant 2015;34:883‐891. [DOI] [PubMed] [Google Scholar]
- 10. Agnoletti G, Ferraro G, Bordese R, Marini D, Gala S, Bergamasco L, et al. Fontan circulation causes early, severe liver damage. Should we offer patients a tailored strategy? Int J Cardiol 2016;209:60‐65. [DOI] [PubMed] [Google Scholar]
- 11. Dijkstra H, Wolff D, Van Melle JP, Bartelds B, Willems TP, Oudkerk M, et al. Diminished liver microperfusion in Fontan patients: a biexponential DWI study. PLoS One 2017;12:e0173149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poterucha JT, Johnson JN, Qureshi MY, O'Leary PW, Kamath PS, Lennon RJ, et al. Magnetic resonance elastography: a novel technique for the detection of hepatic fibrosis and hepatocellular carcinoma after the Fontan operation. Mayo Clin Proc 2015;90:882‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]


